Electron cryomicroscopy (cryoEM)—which won the 2017 Nobel prize—could be made 10 times cheaper by reducing the energy of the microscope and creating better detectors for imaging low energy electrons, according to a proof-of-principle demonstration by scientists at the MRC Laboratory of Molecular Biology (MRC LMB).
They aim to slash the cost of running cryoEM to make it more accessible to scientists all over the world, so they can determine the structures of many more proteins that are important for understanding biology and improving human health.
The development of cryoEM—in which MRC LMB scientists had a leading role, particularly Dr. Richard Henderson who was awarded the Nobel Prize for this work—has dramatically improved the quality and range of biological structures that can be revealed.
However, the technique is still very costly to set up and run—the scientists say that high-end cryoEM installations can cost £5 million to setup with running costs including management in the range of £250,000 annually—putting it out of reach of many laboratories that could potentially benefit from using the microscopes to investigate problems in biology and medicine.
Dr. Christopher Russo from the MRC Laboratory of Molecular Biology, who led this research, said: “Our goal is to make cryoEM available to any laboratory that needs it—so that rapid and simple determination of the structure of any purified biological molecule of interest can become widely available to scientists worldwide.
“The real surprise here is that lowering the energy of the electron beam not only makes the instrument cheaper, but will likely make it better as well. It’s a rare win-win that the physics dictates a way to make things cheaper and better at the same time.”
In the new study, the energy of the beam of electrons that is fired at the specimen was reduced from the current standard of 200 or 300 thousand electron volts (keV) to 100 keV.
Unfortunately, current detectors do not work well at these lower energies, so the team demonstrated a new way to use a different sort of detector, called a “hybrid pixel detector,” to create accurate images of proteins.
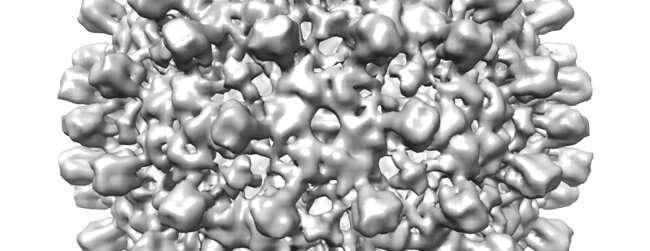
In the week that the prototype microscope was running, they were able to image five specimens (for which structures have previously been determined): hepatitis B virus capsid, bacterial 70S ribosome, catalase, DNA protection during starvation protein, and hemoglobin. From this data they were able to determine 3-D structures for each of the proteins.
Dr. Russo added: “The level of resolution was not a world record, but was as good as more than 90% of the structures determined on all the high-end cryoEM microscopes in the last year. The key thing is to see enough detail to build good molecular models and we demonstrated that here.”
By comparison, before the development of modern cryoEM, determining the structure of the hepatitis B virus capsid took MRC LMB scientists more than a year and the first structure of hemoglobin took many years and was a major milestone in biology.
The detector used by the LMB team in this new lower-energy cryoEM microscope was not purpose-built—it was 32 times smaller than currently used high-energy detectors; the scientists say that a larger detector optimized for working at 100 keV will need to be created. This will take several years and projects are now underway to make bigger, faster and cheaper cameras specifically designed for 100 keV electrons.
Dr. Russo said: “Still, more work needs to be done to turn this lab prototype into a commercial microscope that any biology lab can buy. The main limitation now is the detector—the prototype used a small camera that is similar in pixel number to an early mobile phone camera. We have demonstrated the potential but now we need bigger, faster and more powerful electron cameras for this to really take off—much like modern phone cameras today. Based on this work, several companies and research groups are now working towards this goal and hopefully in a few years this technology will be widely available, as part of a simple cheap microscope that any biology lab will be able to afford.”
The scientists say that a new microscope designed in this way could enable the machine to be both much smaller, simpler and 10 times cheaper to run while still providing the detail required to study molecular structure of proteins.
Another recent study by researchers at the MRC LMB also demonstrated that the lower electron energies could improve the quality of the images over the standard, high energy instruments used today.
Source: Read Full Article
