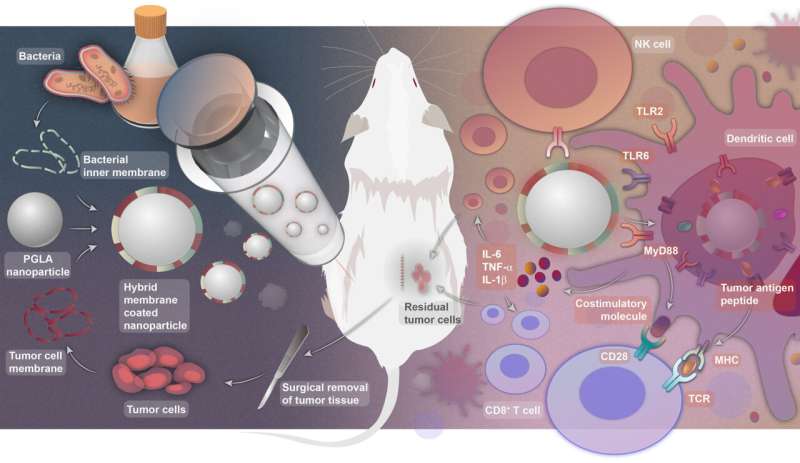
Thanks to the rapid development of nanotechnology, a research team led by Profs. Nie Guangjun, Wu Yan and Zhao Yuliang from the National Center for Nanoscience and Technology (NCNST) of the Chinese Academy of Sciences (CAS) recently designed personalized tumor vaccines based on bacterial cytoplasmic membranes and cell membranes from resected tumor tissue. This work was published in Science Translational Medicine.
Cancer vaccines are an effective anti-tumor therapeutic option that utilize tumor antigens to stimulate patients’ immune response and specifically kill tumor cells. Postoperative recurrence and metastasis after surgery can thus be effectively inhibited by the activated immune system. Therefore, it is important for scientists and clinicians to find the best way to train the patient’s own immune system to find these tumor cells.
Using surgically removed tumor tissue is a very attractive way for making a patient’s own anti-cancer vaccine, since such a vaccine would contain the personalized antigen spectrum of the tumor cells. However, since there is only a small difference between the tumor antigens and the body’s own proteins, the antigens may be recognized as “self” by the patients’ own immune system. As a result, tumor antigens are more likely to induce antigen-specific tolerance than antitumor immunity.
Cancer immunotherapy faces the challenge of how to educate the immune system to distinguish tumor components as ‘non-self.’ Most of the time, bacteria are easily identified as invaders and cleared out by the immune system. Some researchers have tried to use bacteria or their components as adjuvants to enhance immunogenicity. However, nonspecific stimulation of the immune system by bacteria or their components may elicit severe side effects.
For example, lipopolysaccharides—large molecules found in the cell walls of Gram negative bacteria—may cause lethal cytokine storms. Therefore, it is critically important to develop powerful anti-cancer vaccines that educate patients’ own immune systems to find cancer cells but do not induce side effects.
In this work, the research team designed a hybrid membrane nanovaccine for personalized immunotherapy to overcome the challenges described above. The tumor membrane antigens and bacterial inner membrane were fused and displayed on the surface of polymer nanoparticles. Introducing cytoplasmic membranes of E. coli, one of the most common bacteria in the human gut, into the hybrid membrane nanoparticle vaccines induced dendritic cell (DC) maturation, thus activating splenic T cells.
The hybrid membrane-coated nanoparticles represent a novel vaccine strategy that simultaneously delivers antigens and adjuvants to DCs to provoke robust innate and tumor-specific adaptive immune responses. In mouse tumor models, this strategy prevented tumor recurrence, with prolonged tumor-bearing animal survival and tumor-specific, long-term protection against tumor rechallenge.
Source: Read Full Article
