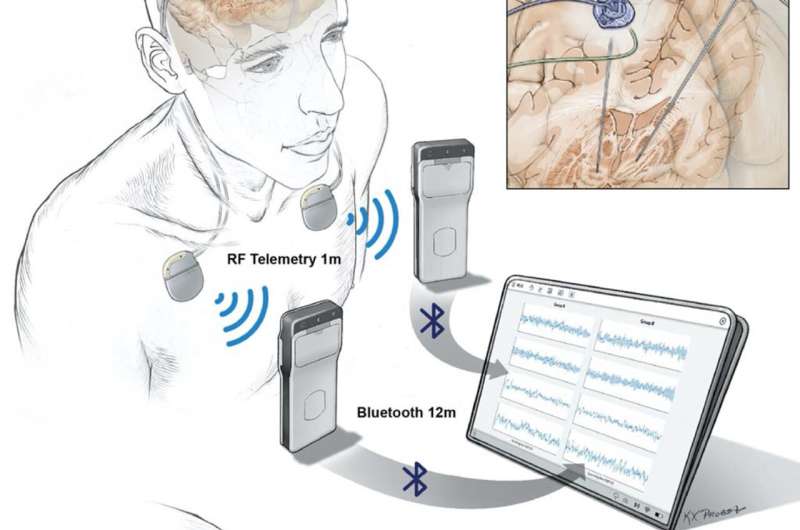
Researchers are now able to wirelessly record the directly measured brain activity of patients living with Parkinson’s disease and to then use that information to adjust the stimulation delivered by an implanted device. Direct recording of deep and surface brain activity offers a unique look into the underlying causes of many brain disorders; however, technological challenges up to this point have limited direct human brain recordings to relatively short periods of time in controlled clinical settings.
This project, published in the journal Nature Biotechnology, was funded by the National Institutes of Health’s Brain Research Through Advancing Innovative Neurotechnologies (BRAIN) Initiative.
“This is really the first example of wirelessly recording deep and surface human brain activity for an extended period of time in the participants’ home environment,” said Kari Ashmont, Ph.D., project manager for the NIH BRAIN Initiative. “It is also the first demonstration of adaptive deep brain stimulation at home.”
Deep brain stimulation (DBS) devices are approved by the U. S. Food and Drug Administration for the management of Parkinson’s disease symptoms by implanting a thin wire, or electrode, that sends electrical signals into the brain. In 2018, the laboratory of Philip Starr, M.D., Ph.D. at the University of California, San Francisco, developed an adaptive version of DBS that adapts its stimulation only when needed based on recorded brain activity. In this study, Dr. Starr and his colleagues made several additional improvements to the implanted technology.
“This is the first device that allows for continuous and direct wireless recording of the entire brain signal over many hours,” said Dr. Starr. “That means we are able to perform whole brain recording over a long period of time while people are going about their daily lives.”
The implications of this type of recording are significant. The brain activity patterns (neural signatures) normally used to identify problems such as Parkinson’s disease symptoms have traditionally been recorded in clinical settings over short periods of time. This new technology makes it possible to validate those signatures during ordinary daily activities.
“If you ever hope to use in-hospital recordings to modify a disease state through adaptive stimulation, you must show that they are also valid in the real world,” said Dr. Starr.
Another advantage to recording over long periods of time is that distinct changes in brain activity (biomarkers) that could predict movement disorders can now be identified for individual patients. Ro’ee Gilron, Ph.D., a postdoctoral scholar in Dr. Starr’s lab and first author of this study, explained that this allows for a level of customized DBS treatment that was impossible to achieve previously.
“Because we are able to build a biomarker library for each patient, we can now program each DBS unit according to a patient’s individual needs,” said Dr. Gilron. “This includes personalized stimulation programs that adapt as the patient’s needs change throughout the day.”
One important consideration that arises is the ethical implication of (nearly) all-day brain recording. Since its beginning, the NIH BRAIN Initiative has recognized the importance of addressing potential ethical considerations pertaining to the development and use of devices that record or modulate brain activity. For instance, the NIH BRAIN Neuroethics Working Group is a group of experts in neuroethics and neuroscience that serves to provide the NIH BRAIN Initiative with input relating to neuroethics—a field that studies the ethical, legal, and societal implications of neuroscience. Alongside funding for neurotechnology research, the Initiative also funds research on the ethical implications of advancements in neurotechnology.
“We have had patients approach us with concerns regarding privacy,” said Dr. Starr. “Although we are not at the point where we can distinguish specific normal behaviors from brain activity recording, it is an absolutely legitimate concern. We have told patients to feel free to remove their wearable devices and to turn off their brain recordings whenever they engage in activities they would like to keep private.”
The patients were also invited to participate in NIH BRAIN Initiative-funded neuroethics projects looking to identify concerns about this new technology (MH114860). In addition, individuals who opted out of the implant project were interviewed about their decision. As recommended by a recent BRAIN 2.0 neuroethics report, this information will be used to develop ethical guidelines and protocols for future projects to achieve a healthy balance between discovery and privacy.
One unforeseen benefit of this study was that, because it required little to no direct contact with clinicians following surgery, it was ideally suited for the social distancing that is crucial during the COVID-19 pandemic. The technologies used for remote patient monitoring and telehealth were originally designed for the convenience of study subjects, but they have broader applications to other research projects that have been stalled due to COVID-19.
“The technologies we developed and used to communicate and work remotely with our patients can also allow those who do not live close to a clinic to receive ‘over the air’ updates for their devices and telehealth visits from their neurologists as they manage increasingly complex DBS devices,” said Dr. Gilron.
Source: Read Full Article
