Metastasizing breast cancers typically seek out the bones, lung, and brain. Brain metastases are especially dangerous; many women survive for less than a year after diagnosis. How is the cancer able to get past the blood brain barrier? And can it be blocked?
Those questions led Ph.D. candidate Golnaz Morad, DDS, and her mentor Marsha Moses, Ph.D., to conduct an in-depth investigation of exosomes, also known as extracellular vesicles or EVs, and their role in breast-to-brain metastasis. Their surprising findings appear in the journal ACS Nano.
“Golnaz was able to identify the mechanism by which EVs pass through the blood brain barrier and provide a ‘niche’ so that breast cancer cells can metastasize to brain,” says Moses, who directs the Vascular Biology Program at Boston Children’s Hospital and whose lab is interested in women’s cancers.
Now that they know the mechanism, Moses and Morad hope to identify therapeutic targets that could stop brain metastases from happening.
EVs and cancer
Simply put, EVs are tiny bubbles released by cells, encapsulating chemical messages they wish to convey. In the case of cancer cells, EVs carry factors that help create a more hospitable environment for both the primary tumor and its metastases, as Moses and Morad detailed recently in a review article. Primary tumors can secrete EVs into the circulation, allowing them to travel to distant organs and help spread the cancer.
“The main question we had was, how can EVs reach the brain in the first place?” says Morad. “The blood brain barrier doesn’t allow anything larger than 400 daltons to passively get into brain tissue. Exosomes are more than two thousand times larger than the size cutoff limit.”
Hijacking transcytosis
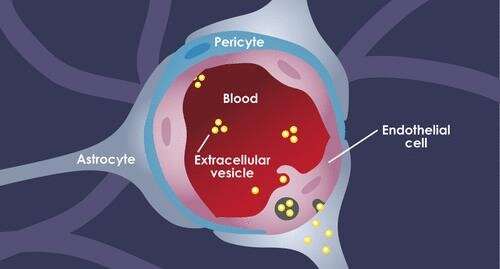
The blood brain barrier (BBB) is a complex structure made up of three kinds of cells. EVs traveling in the blood first encounter tightly joined brain endothelial cells. Pericytes comprise the next layer, followed by astrocytes (the “feet,” which connect to the cell bodies). Crosstalk between the cells circles the wagons even tighter: Astrocytes and pericytes send cues to the endothelial cells to tighten up the junctions.
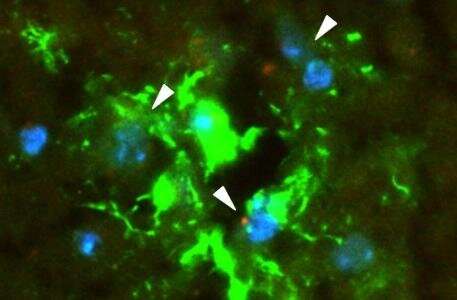
Morad, Moses, and colleagues worked with several models of the BBB, including in vitro microfluidic models of the BBB (“BBB on a chip”), as well as static in vitro models and live zebrafish and mouse models. The labs of Donald Ingber, MD, Ph.D., and Leonard Zon, MD, collaborated in the development of the BBB-on-a-chip and zebrafish models, respectively. The Moses Lab also collaborated with the lab of Christopher Carman, Ph.D., of Harvard’s T.H. Chan School of Public Health for the high-resolution imaging conducted in this study.
Each model and technique provided its own insights. Bottom line, Morad and Moses showed, for the first time, that rather than squeezing in between the cells in the BBB to get into the brain, the EVs trick the endothelial cells into taking them up. Using a standard biological pathway called transcytosis, the cells simply engulfed the EVs, bringing them inside and releasing them into brain tissue like so many Trojan horses.
“EVs can also manipulate endothelial cells to facilitate their own transport across the BBB,” says Morad. “They hijack the pathways involved in the uptake and sorting of molecules and change regulation of the pathways.”
Ongoing work in the Moses Lab shows that once the EVs have breached the barrier, they trick astrocytes into sending signals to the surrounding environment, making it more receptive to tumor growth. An inventory of these signals, using mass spectrometry and other techniques, identified some that are known to degrade the network of proteins providing structural and biochemical support to brain cells.
EVs as anticancer delivery vehicles?
For the study, Morad had to develop special methods to harvest the EVs in quantity and to verify their identity.
“They are remarkable little vehicles, but it is very challenging to get enough of them,” says Moses.
Having discovered what EVs do to help breast cancer metastasize to the brain, the Moses Lab hopes to turn the tables—and use EVs (or synthetic versions) to deliver anticancer drugs that home to the metastatic site. That work is ongoing.
Source: Read Full Article
