Cardiologist Nisha Parikh, M.D., MPH, discusses what we know so far about COVID-19’s impact on the body’s cardiovascular system, from affecting the heart’s rhythm to impairing its ability to pump blood throughout the body.
We used to think of COVID-19 as just a lung disease. What were the first signs that this view wasn’t quite accurate?
One of the first indications that this virus affected the cardiovascular system was an early report about how SARS-CoV-2, the virus that causes COVID-19, enters a cell using the receptor involved in the system that regulates the body’s blood pressure and level of water and salts, which is known as the renin-angiotensin-aldosterone system. That article definitely got my attention and the attention of my colleagues. This receptor is called the ACE2 receptor, and its involvement might be behind why people with obesity, diabetes, and high blood pressure tend to get more severe forms of COVID-19.
Although we haven’t quite unraveled that story, I think we are going to learn more.
What do we know so far about how COVID-19 affects the heart in the short term?
There are many ways COVID-19 can affect the heart during the initial period when someone gets the infection, particularly in the first few weeks. These effects can include new or worsened problems pumping blood effectively, inflammation of the heart muscle, and inflammation of the membrane around the heart. It should be noted that other viruses also can cause these conditions.
We determined this based on deaths from COVID-19 that we know about. But there’s also been a spike in deaths occurring outside the hospital nationwide since this spring, including people being found dead at home from unknown causes. It makes you wonder how many of these out-of-hospital deaths were really COVID-19-related cardiovascular deaths.
Finally, we are also seeing effects from COVID-19 that may not be directly due to the virus but are related to people not obtaining appropriate medical treatment for a heart problem, often because they have delayed seeking medical care for hours, days, or even weeks after potential heart attack symptoms began. This has led to some really serious complications we hadn’t seen in some time because we now have such good treatments for heart attacks that are detected early enough. These issues include potentially deadly problems such as holes in the heart muscle and tears in the heart valves.
The other thing my colleagues and I have seen when caring for patients admitted to the hospital is a higher incidence of a condition called Takotsubo cardiomyopathy, which is heart dysfunction that occurs when someone is under tremendous stress. These days, that stress might be from social isolation, from having loved ones fall ill, or from having lost loved ones. But that trend is anecdotal; we don’t really know the scope of the problem yet.
What symptoms are you seeing among people who had COVID-19 in the past?
We are starting to see more patients with cardiovascular symptoms ranging from chest pain to palpitations to presyncope or syncope—which is feeling lightheaded, like you’re going to faint—that are often accompanied by neurologic symptoms such as brain fogginess, headaches, numbness, or other sensations in various parts of the body. These are part of a constellation of symptoms that folks in this so-called COVID-19 long-hauler category are experiencing.
Many of the symptoms seem to be tied to the nervous system and in many ways may share similarities with cardiovascular diseases we have known about for some time, such as postural orthostatic tachycardia syndrome. POTS, as it’s known, is characterized by an abnormal increase in heart rate when standing up and can lead to dizziness, fainting, and other debilitating symptoms. We would like to look further into whether this collection of COVID symptoms we are seeing is POTS.
Are signs of heart problems showing up in people of all ages?
Yes, we have seen young, middle-aged, and older adults develop heart issues. Of the two main studies of heart function among patients who’d had COVID-19—including infections that were asymptomatic—the average age of participants in one was 49 years old; the other was a study of competitive college athletes who were an average of 19.5 years old. Everyone working to answer questions related to heart function and COVID-19 agrees that we should be taking these studies as preliminary and certainly not definitive; more work needs to be done.
What initial testing do you perform on patients whose hearts may have been harmed?
The tests I start with are an ultrasound of the heart, known as an echocardiogram, as well as some sort of heart rhythm monitoring to look for concerning rhythm issues, especially if a patient is experiencing palpitations or lightheadedness. What we are looking for on the heart ultrasound is whether the heart’s pumping function has been damaged. One of the things we noticed early in the pandemic was the presence of inflammation of the heart muscle, which could even progress to problems with the heart muscle’s function, so we keep an eye out for those types of issues. We are also looking for any evidence of an abnormality of the right side of the heart, which can happen if you have a lung injury or a blood clot in the lungs, both of which people with COVID-19 often have.
What have these tests revealed?
Many of the echocardiograms—the heart ultrasounds—have been normal, but some have shown that the heart is having problems pumping blood to the rest of the body, compared to what a normal heart can do. Studies have shown a decreased degree of what is called global longitudinal strain, which can be an early warning sign of an impending issue with the heart’s pumping ability.
Another concerning thing we have noticed in many patients who have recovered from the infection is inflammation or scarring in the heart muscle. A recent study, for example, showed that patients who had recently recovered from COVID-19—even if it was an infection without symptoms—were significantly more likely to have evidence of heart muscle damage than people who hadn’t had COVID-19.
How does COVID-19 cause long-term effects on the heart?
COVID-19 probably directly or indirectly affects heart muscle cells and other heart tissue, even among patients who did not have signs or symptoms of COVID-19. What that means over the long term is not clear. These effects may continue to be subtle—maybe they’ll never lead to symptoms or problems—or they could lead to a change in the way the heart muscle functions.
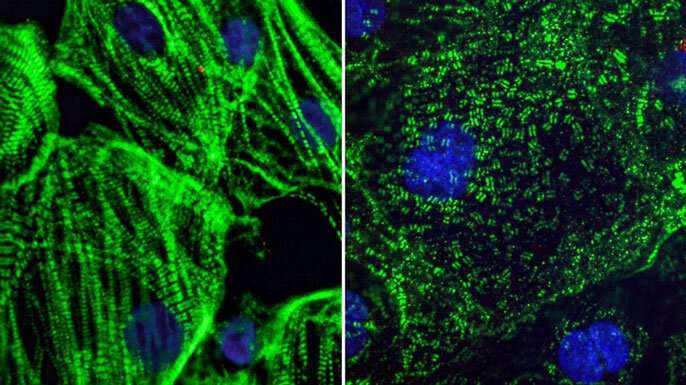
A recent study from the Gladstone Institutes, one of UCSF’s partners, showed that infecting heart cells with COVID-19 caused the building blocks of muscles to appear splayed every which way, rather than arranged in an organized line as they are in healthy muscle. These findings could explain some of the issues we are seeing in the heart long after a person recovers from COVID-19. But it’s important to note that one would have to translate those results into a human model, since the study was in cell culture.
What has emerged about how COVID-19 might attack the body’s circulatory system beyond the heart?
It seems the virus directly infects the lining of blood vessels, known as the endothelium, which may be why there are really high rates of clots, such as in the lungs and legs, among patients hospitalized with COVID-19. It’s not clear what physiologic process is driving the clots—whether it’s a direct effect of the virus or whether being sick and immobilized contributes to it as well. Researchers at the National Institutes of Health and elsewhere are studying how to prevent blood clots among patients with COVID-19.
What should we do next to learn more about the long-term effects of COVID-19 on the heart?
Source: Read Full Article
