Interaction between the gut microbiota and the immune system is important for host physiology and susceptibility to disease, but also for the efficacy of e.g., cancer immunotherapies. A multidisciplinary research team have now discovered that specific probiotic bacteria shape the intestinal microbiome by affecting B lymphocytes in the Peyer’s patches to induce, produce and release IgA following trafficking to the mucosa of the small intestine and colon.
“These findings have major implications for therapeutic targeting of the microbiome to reduce inflammation and improve drug treatment efficacy,” says Mia Phillipson, Professor at Uppsala University’s Department of Medical Cell Biology and corresponding author.
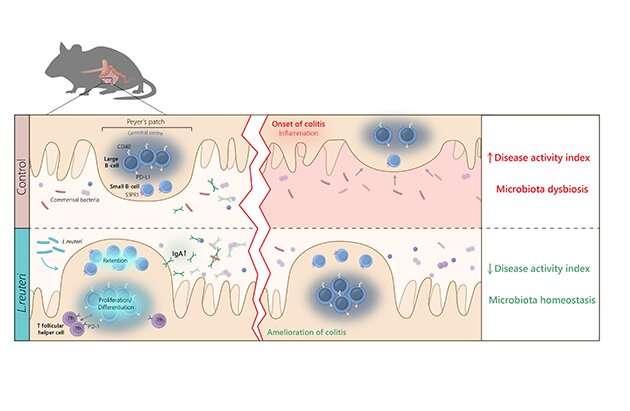
Intestinal immune cells accumulate in lymphoid tissues of the small intestine, also known as “Peyer’s patches,” which offer unique opportunities for bacteria—immune cell interactions. Strategies to alter the intestinal microbiota for health benefits can involve dietary addition of probiotics, bacteria that provide health benefits for the host.
Such attempts have been tried with some success and associations between orally administrated probiotics and improved intestinal health and protection against colitis have been demonstrated. However, the fundamental understanding of how minute amounts of ingested probiotic bacteria can improve gut homeostasis while transiting the gastrointestinal system is completely lacking.
A new study published by researchers at Uppsala University, SciLifeLab and Swedish University of Agricultural Sciences, SLU, found that the immune cells of Peyer´s patches both sense and transmit probiotic signals important for shaping the microbiome of small intestine and colon. The study shows that Limosilactobacillus reuteri-treatment of healthy mice increased both the numbers and strength of effector functions of distinct subsets of large and small B lymphocytes in the Peyer’s patches, and resulted in IgA induction and production in a PD-1-dependent manner. Consequently, IgA-producing B cells populating the mucosa altered the intestinal microbiome, which reduced inflammatory symptoms and improved the composition of the microbiota in a model of colitis.
Source: Read Full Article
