Millions of people, including athletes who play contact sports, members of the military and victims of domestic violence, are exposed to repetitive head impacts (RHI), which is the primary risk factor for developing chronic traumatic encephalopathy (CTE). Symptoms of CTE often manifest years to decades after exposure to RHI and very little is known about what happens in the brain in the interim.
The brains of people who die with CTE are marked by the accumulation of a protein called tau, the same protein found to aggregate in Alzheimer’s disease (AD) brain. The amount of tau pathology in CTE correlates with severity of disease, where early-stage brains have very little pathology and late stage show severe, widespread involvement.
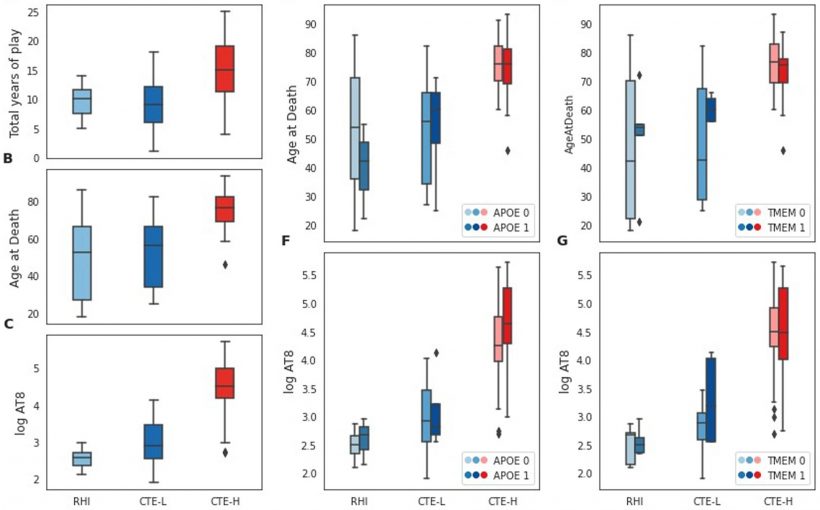
The amount of RHI exposure, which for athletes can be measured in terms of the number of years they played a violent sport, as well as genetic risk variants influence the extent of tau pathology and associated disease severity. However, the molecular and genetic mechanisms that underly the development disease, and to what extent those effects are consistent throughout disease progression, are poorly understood.
To address these questions, researchers from Boston University Chobanian & Avedisian School of Medicine conducted genetic analysis of the largest collection of post-mortem CTE brains, primarily from professional athletes, donated to the BU CTE Brain Bank. They found evidence that early and late CTE brains are similar in some ways but dramatically different in others.
In particular, neuro-inflammation and neuronal stress are strongly implicated in disease, albeit to different extents and in different directions depending on the severity of disease. This is the first study to show that the molecular pathways involved in early CTE are different from those involved in late-stage disease.
“A better understanding of the early CTE disease process may lead to more informative diagnostics, biomarkers and ultimately therapies,” said co-corresponding author Adam Thomas Labadorf, Ph.D., assistant professor of neurology. “In addition, since the type of pathology found in the brains of people with CTE is similar to that found in AD, a better understanding of how the brain responds to this kind of pathology in CTE is likely to better inform our understanding AD as well.”
The researchers studied the prefrontal cortex tissue from 76 individuals (66 CTE, 10 control) who donated their brains to the BU UNITE Brain Bank. The sample set contained brains that spanned the full range of disease severity, affording the researchers the unique opportunity to see whether the gene expression in people with early stage CTE differ from those with late stage.
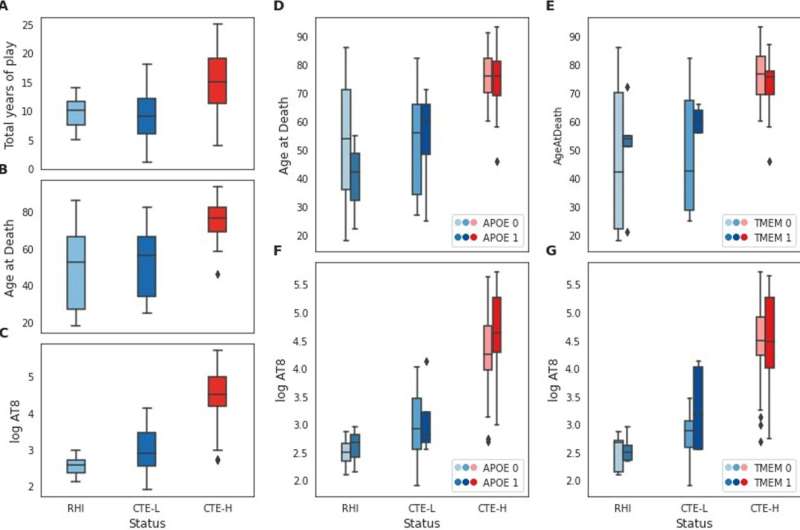
The researchers generated gene expression data for each individual and then performed bioinformatic and statistical analyses of the different subsets of these samples to look for gene expression patterns that are associated with different clinical, histological and genetic markers that are relevant to CTE. They then identified genes and biological processes associated with total years of play as a measure of exposure, amount of tau pathology present at time of death, and the presence of APOE and TMEM106B risk variants.
The researchers found substantial gene expression changes were associated with severe disease for most of these factors, primarily implicating diverse, strongly involved neuro-inflammatory and neuro-immune processes. In contrast, low pathology groups had many fewer gene expression changes and neuroimmune or inflammatory processes implicated and showed striking differences for some factors when compared with severe disease.
According to the researchers, if the active disease process in early disease differs substantially from late-stage disease, this could have important implications for both diagnostic and therapeutic targets.
“This might explain why therapeutic targets identified from late-stage human tissue have largely failed to influence disease progression in clinical trials for many neurodegenerative diseases. In addition, if there are distinct markers of early disease progression that are absent in late disease, this would provide an opportunity to explore different diagnostics and biomarkers that we otherwise wouldn’t know to look for,” explained co-corresponding author Thor Stein, MD, Ph.D., a neuropathologist at VA Boston Healthcare System and associate professor of pathology and laboratory medicine.
These findings appear online in the journal BMC Medical Genomics.
More information:
Adam Labadorf et al, Inflammation and neuronal gene expression changes differ in early versus late chronic traumatic encephalopathy brain, BMC Medical Genomics (2023). DOI: 10.1186/s12920-023-01471-5
Journal information:
BMC Medical Genomics
Source: Read Full Article