Actomyosin is a protein complex composed of actin and myosin. It is found in muscle fibers where it plays a role in muscle contraction.
Credit: Tsvetkov Maxim/Shutterstock.com
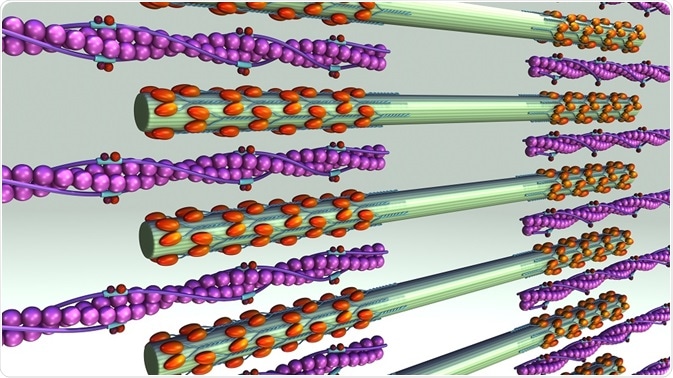
Actin is one of the most abundant proteins in eukaryotes. It exists in two forms: monomeric globules known as G-actin and polymeric filaments called F-actin. Myosin molecules have three main domains, the head, neck, and tail. The head domain binds to F-actin and uses ATP hydrolysis to yield energy to move itself along the actin filament.
Actomyosin and muscle cells
Force is generated in cells by protein-based machines located in the cytoskeleton. These machines capture chemical energy to perform mechanical work. Molecular interactions between F-actin and non-muscle myosin II control the distribution of mechanical force for changes in cell shape, division, and migration, as well as the contraction of smooth muscle.
Polymers of F-actin form the scaffold for myosin II motors and other proteins. Actin filaments have two ends. One end is barbed and fast-growing and the other is pointed and slow-growing. In order for contraction to occur, the coordinated activity of myosin II within the F-actin scaffold is required.
Myosin II motors are contained in myosin filaments, which can range from a few dozen heads to hundreds of heads. Myosin II filaments move F-actin filaments toward their barbed ends, causing contraction or extension of the actin filaments.
This movement is caused by ATP-hydrolysis powered myosin head dissociation from the actin filament, followed by a conformational change that leads to a 5 nm change in head position. The head now binds to a new position on the actin filament, and then returns to its original bent position, thus dragging the actin forward one step.
The walking motion of the myosin heads along the actin strand is responsible for muscle contraction, bringing the ends of the sarcomeres closer together and shortening the length of the muscle fiber.
Accessory actin-binding proteins
Accessory actin-binding proteins modulate the mechanics of the F-actin network. Some of these proteins include α-actinin, filamen, and tropomyosin. Actin-binding proteins also modify the coupling of actin to the plasma membrane. In striated muscle, actomyosin is found in crystalline arrays. These are called sarcomeres.
When myosin pulls actin filaments together, the sarcomere length is reduced. In non-muscle and smooth muscle cells, actomyosin is not organized into sarcomeres. Instead, smooth muscle contains loosely organized actomyosin bundles.
During cell division, an actomyosin ring is formed that drives cytokinesis. In adherent non-muscle cells, actomyosin forms a contractile network and different types of bundles, some of which have similar properties to sarcomeres. Contractile actomyosin arrays in non-muscle and smooth muscle are dynamic, and may remodel under stress.
Credit: sciencepics/Shutterstock.com
Actomyosin networks are mechanosensors
Contractile actomyosin networks can also function as sensors. Actin networks are flexible under low stress, but when stress is increased, their stiffness increases by as much as a hundred fold. When myosin II motors are present in the actin network, they create stresses in the system and stiffen the network. These changes in the stiffness of the actomyosin network are believed to be important in responding to external forces.
Actomyosin circulates cytoplasm
Actomyosin networks create motions that deform the actin cortex, creating random-seeming movements. This results in motion of organelles and proteins within the network, leading to active diffusion, or ‘stirring’ within the cell. This stirring effect enhances intracellular transport and intracellular signaling.
Actomyosin ring
The actomyosin ring is a structure that forms during cell division. It forms perpendicular to the axis of the spindle apparatus. The ring contracts with centripetal growth of the membrane to facilitate cell division. The actomyosin ring forms in four stages. In the first stages: specification of division site, ring formation, ring constriction, and subsequent ring disassembly.
Sources:
- Regulation of actomyosin and contraction in smooth muscle
- Forcing cells into shape: the mechanics of actomyosin contractility
- Actomyosin ring formation and tension generation in eukaryotic cytokinesis
- Actin, myosin, and cell movement
Further Reading
- All Cell Migration Content
- Microtubule Motor Proteins
- Selectins
- Structure and Function of Proteoglycans
- Confocal Reflection Microscopy for Cell Migration Studies
Last Updated: Feb 26, 2019

Written by
Dr. Catherine Shaffer
Catherine Shaffer is a freelance science and health writer from Michigan. She has written for a wide variety of trade and consumer publications on life sciences topics, particularly in the area of drug discovery and development. She holds a Ph.D. in Biological Chemistry and began her career as a laboratory researcher before transitioning to science writing. She also writes and publishes fiction, and in her free time enjoys yoga, biking, and taking care of her pets.
Source: Read Full Article
