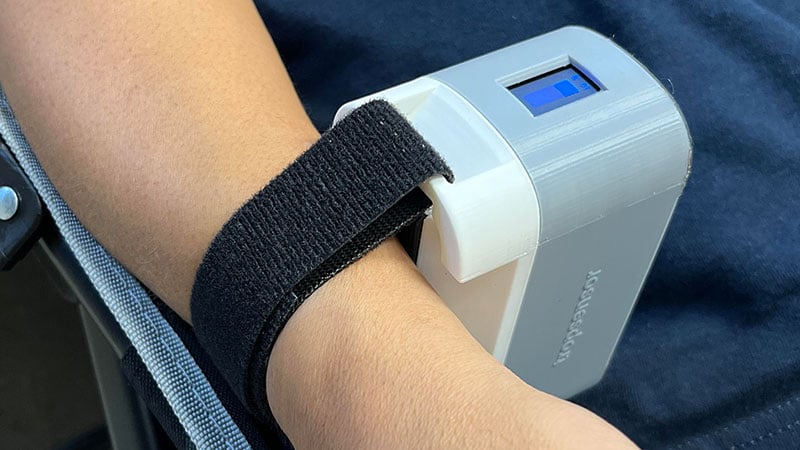
The use of a transdermal sensor for the measurement of cardiac troponin may have a role in the diagnosis of acute myocardial infarction (MI), a new study suggests.
The device, a wrist-worn sensor that uses infrared light to detect the presence and concentration of cardiac troponin-I in the blood through the skin, was found to predict levels with 90% accuracy within 5 minutes.
“This is an exciting opportunity because it increases our capability for early diagnosis of heart attacks in both community settings and in acute care environments,” said Partho P. Sengupta, MD, professor of cardiology at Rutgers Robert Wood Johnson Medical School in New Brunswick, New Jersey, and the study’s lead author.
“There’s still a lot of work to be done, but this approach could potentially address access and prioritization issues, for example, by shortening the time to triage, or being used by emergency responders to plan the patient’s journey before they even arrive at the hospital.”
Sengupta presented the study at the recent American College of Cardiology (ACC) Scientific Session/World Congress of Cardiology (WCC) 2023.
The results were also simultaneously published online in the European Heart Journal – Digital Health on March 6.
Commenting on the study at an ACC press conference, Jim Cheng, MD, Weill Cornell Medicine, New York, who was not involved in the research, said the technology was “very exciting.”
He added that this could “usher in a new frontier for biosensors” and “could potentially result in improved management of patients with coronary artery disease and maybe also those with congestive heart failure.”
Noting that the wait for troponin blood test results in the emergency department can lead to delays in diagnosis, Cheng said: “This study shows the feasibility of this wrist-worn approach that can potentially provide very rapid results without the need for blood draws and which showed excellent discriminative ability in this particular set of patients.”
In addition to allowing more rapid triage of patients in the emergency department, Cheng pointed out that this technology could also be used in the ambulance. Paramedics could call the hospital in advance to make arrangements for immediate cardiac catheterization.
He also noted that because there is a lag between the timing of myocardial injury and troponin elevation, the ability to continuously measure troponin with a device like this could allow early, rapid detection of injury, leading to earlier treatment.
Cheng cautioned, however, that this was a preliminary study that involved a relatively small number of patients and that there is need for additional refinements of the technology.
“It needs to be tested in a wide variety of patient populations in different settings, and we don’t know yet how it would perform in the real-world setting, which would include patients with noncardiac chest pain,” he added.
Marker of Myocyte Necrosis
In his presentation, Sengupta explained that measurement of cardiac troponin levels, as a marker of myocyte necrosis, is routinely recommended for patients who present in the emergency department with suspected acute coronary syndrome (ACS). For these patients, it is recommended that blood draws be conducted within a turnaround time of 60 minutes.
But turnover times for laboratory tests vary widely, and meeting recommendations is challenging in overcrowded acute care settings. The problem is made worse by staff shortages and reduced hospital resources. A potential way to avoid delay is through use of a point-of-care tool that can confirm diagnoses and that can be used to risk-stratify patients with suspected MI.
The new device, known as a transdermal infrared spectrophotometric sensor, is in development by RCE Technologies.
The current observational study, conducted at five sites in India, compared the transdermal sensor with the regular blood tests for assessing troponin-I levels at a single random time point during the hospital stay for 239 ACS patients whose conditions had been clinically stabilized.
All patients wore the wrist-based sensor and underwent a blood draw to assess troponin-I levels. The final diagnosis of MI (with or without ST elevation) and unstable angina was adjudicated using electrocardiography, cardiac troponin level, echocardiography (regional wall motion abnormality), or coronary angiography.
The researchers used data from the first three sites to train the machine-learning model and then used the remaining two sites to test the model’s accuracy.
Results showed that the transdermal sensor predicted elevated high-sensitivity cardiac troponin-I levels with 90% accuracy.
The area under the receiver operating curve, which quantifies how well the transdermal sensor discriminated between those with and those without elevated troponin-I levels, was 0.90 (95% CI, 0.84 – 0.94) with a sensitivity of 0.86 and a specificity of 0.82.
In addition, the findings correlated well with clinical evidence of MI; patients with abnormal troponin levels, as measured by the device, were about four times more likely to have an obstructed artery compared to those with a negative troponin result, as measured by the device.
“With this level of accuracy, if you use this device and it comes out positive, you’re fairly sure this patient can be admitted for fast tracking diagnostic tests, treatment, and intervention,” Sengupta said.
He cautioned that more studies are needed to further validate and refine the system, including studies to determine whether biological variability ― such as differences in skin tone, wrist size, skin health, or other factors ― could affect the device’s performance.
In addition, the researchers plan to study whether including the detected troponin value (rather than simply indicating whether the measured level met or did not meet a threshold value) or providing continuous measurements could enhance the device’s usefulness in clinical settings.
Sengupta noted that the wearable sensor technology could potentially be adapted for aiding triage and diagnosis for a variety of cardiovascular diseases and other health problems.
“Transdermal infrared-based techniques open up a tremendous potential for bloodless biomarker assessment. We have started with troponin, but the journey is going to continue, because it is possible to use this technology for other biomarkers. This is just the start,” he commented.
Discussing the study at the ACC late-breaking clinical trial session, James Januzzi, MD, Massachusetts General Hospital, Boston, described the transdermal sensor as “an innovative approach to a very prominent issue that we face which is the challenge of sampling troponin for our patients.”
He added that the importance of this research “extends far beyond the walls of the emergency department,” in that being able to measure troponin in this way in other areas, such as for patients with chronic heart failure to assess cardiac remodeling and for patients with symptoms of ischemic heart disease before they reach hospital, “could potentially be of great value.”
The study was funded by RCE Technologies Inc. Sengupta serves as an advisor to RCE Technologies and has equity and stock options in the company.
American College of Cardiology (ACC) Scientific Session/World Congress of Cardiology (WCC) 2023: Late Breaking Clinical Trials. Presented March 6, 2023.
European Heart Journal – Digital Health. Published online March 6. Full text
For more from the heart.org | Medscape Cardiology, follow us on Twitter and Facebook.
Source: Read Full Article