Sensory inputs, including visual, auditory, and somatosensory inputs from the environment, play a crucial role in infant brain development. Vision (light), the most important perception of mammals, has been identified for promoting synaptogenesis, one of the hallmarks of brain development, in multiple areas of the brain. However, the neural mechanism regulating this phenomenon and the lifelong effects on cognition and learning ability remain unknown.
The research team led by Prof. Xue Tian and Bao Jin from the Department of Life Science and Medicine at the University of Science and Technology of China (USTC) of Chinese Academy of Sciences identified the neural mechanism and lifelong effect of light-promoted early brain development in mammals. The results were published in Cell.
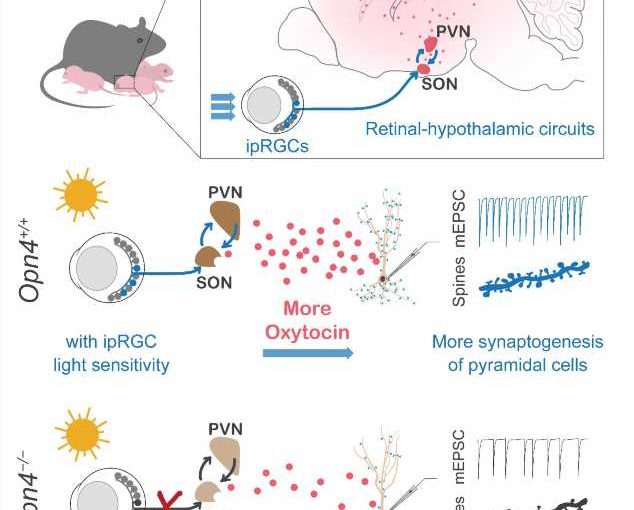
Visual perception begins with the retina. There are three main types of photoreceptors in the mammalian retina: rods, cones, and intrinsically photosensitive retinal ganglion cells (ipRGCs). Unlike the classical imaging visual photoreceptor rods and cones that mediate visual image encoding, ipRGCs are specifically activated by blue light through melanopsin, the opsin encoded by gene Opn4, and mainly mediate non-imaging visual functions (NIV) such as light entrainment of circadian clocks, pupillary light reflex, and mood regulation. Developmentally, ipRGCs respond to light much earlier than rods and cones and mediate the earliest light perception in mammals, suggesting that ipRGCs may play a role in light-promoting brain development.
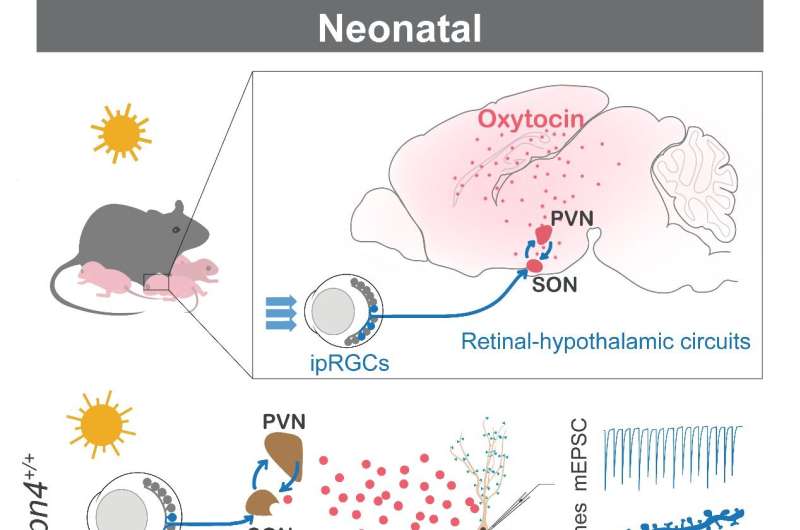
In this study, the researchers first found that compared to control pups (Opn4+/+), neonatal mice lacking the photosensitivity of ipRGCs (Opn4-/-) had reduced frequency of mini-excitatory postsynaptic currents (mEPSCs) and number of dendritic spines of pyramidal neurons in multiple sensory cortex and hippocampus, while those deficits of synaptogenesis were observed in another experimental group reared in the dark from birth. Furthermore, by re-expressing melanopsin in the ipRGCs of Opn4-/- neonatal mice, the cortical and hippocampal synaptogenesis in the cortex and hippocampus were significantly enhanced. This result revealed that light sensation through ipRGCs mediate the light-promoted brain synaptogenesis of neonatal mice at early brain development.
By mass spectrometry, the researchers further identified the neuropeptide oxytocin as the signaling molecule for ipRGCs-mediated light-promoted cortical and hippocampal synaptogenesis. They confirmed the projection of ipRGCs to supraoptic nucleus (SON) and showed retinal ganglion cells connect with oxytocin neurons in both SON and paraventricular nucleus (PVN) in pups. This projection leads to the activation of oxytocin neurons in both SON and PVN upon light sensation mediated by ipRGCs, increasing the concentration of oxytocin in cerebrospinal fluid which promotes the synaptogenesis.
To further identify whether the observed deficits in cortical synaptogenesis at early developmental stages have any long-term impacts on adult mice, the researchers applied a sound discrimination task to test the learning ability of two-month-old mice. Compare to control mice, the Opn4-/- mice showed deficit in learning speed, meanwhile this deficit can be rescued via re-expressing melanopsin, or artificial activation of oxytocin neurons in SON from birth.
Source: Read Full Article