Lung cancer claims more lives each year than any other type of cancer worldwide. According to the World Health Organization, an estimated 1.8 million people died of lung cancer in 2020. Current treatments rely on one of two avenues: generalized chemotherapy that inflicts harsh side effects on cancer patients, or targeting of tumors with very specific mutations that may not apply to many patients—both of which make fighting the disease challenging.
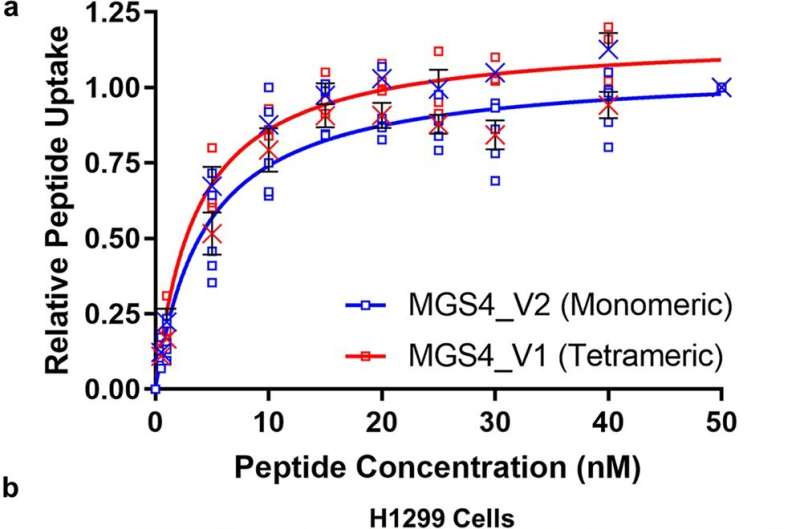
Researchers at SRI International have designed and optimized a new peptide—a molecule that contains two or more amino acids—to act as a delivery vehicle for drugs to treat non-small cell lung cancer, which accounts for the majority of lung cancer cases. This peptide, highlighted in a recent study published in Nature Communications Biology, can carry large anti-cancer drugs and successfully target cancerous cells, binding to them and triggering a process to draw the peptide and its cargo inside.
“With our peptides, we can start to deliver large cargos inside of a cell that couldn’t otherwise pass through the cell membrane,” said Kathlynn Brown, vice president of drug delivery systems at SRI’s Bioscience division and lead author on the paper. “It opens the door to basically dragging therapeutics into the inner workings of tumor cells while avoiding healthy cells, minimizing the potential side effects.”
Currently, most similarly targeted cancer drugs are delivered by antibodies, which bind to specific receptors on the surface of a cancer cell. But there are several advantages to using peptides instead of antibodies. First, peptides are significantly smaller molecules, which means that they can penetrate deeper into a tumor. Second, peptides can be put together chemically, while antibodies must be produced biologically in cells. The chemical process is faster, less expensive and gives researchers more precise control over the final result.
“Because we make them chemically, we have a lot of flexibility in how we incorporate the drug,” Brown said. “We can put on whatever drug or cargo we want, we will know exactly where it is, and we can modify that with tools we have in the lab.”
To find a peptide with the right set of traits, Brown and her colleagues use a proprietary selection process to sift through a library of billions of potential options. In this case, they needed a peptide that could successfully seek out and bind to cancer cells while leaving healthy cells alone; could trigger biological processes to rapidly draw the peptide and its cargo into the cell; and wouldn’t degrade while circulating through the body.
“We don’t try to target a particular cell surface marker to begin with,” Brown said. “We do what’s referred to as an unbiased selection, which basically means that we’re going to let the cells tells us the best answer. We’re not going to try to outsmart mother nature.”
Once Brown and her team identified a peptide with the right traits, known as MGS4, they tweaked it to be as effective as possible and tested it with a toxic protein called saporin, which can’t get into cells very effectively on its own. They found that MGS4 successfully delivered saporin to lung cancer tumors in mice, and after 18 days of treatment, tumors in mice given saporin attached to MGS4 were half the size of tumors in untreated mice or in mice given saporin by itself.
Their work demonstrates that MGS4 could be a valuable tool in developing targeted therapeutics for lung cancer. And because it can carry a variety of drugs, the researchers are just beginning to explore its potential to facilitate treatments.
“This protein toxin is our first therapeutic concept, but what’s really important is that this peptide is able to deliver large, toxic, biologic therapies inside of a tumor cell,” Brown said. “We’re really hoping that we can take this peptide and use it in multiple different applications with multiple different therapeutics.”
More information:
Curtis A. Allred et al, Tumor-specific intracellular delivery: peptide-guided transport of a catalytic toxin, Communications Biology (2023). DOI: 10.1038/s42003-022-04385-7
Journal information:
Communications Biology
Source: Read Full Article