Ever since 19th-century neuroanatomist Santiago Ramón y Cajal discovered the brain’s beautifully complex neurons—what he referred to as “the mysterious butterflies of the soul”—neuroscientists have sought to characterize the hundreds or thousands of varieties of brain cells and reveal how they work together. It’s a lofty goal, but one that would greatly help neuroscientists finally understand the intricate web of the brain’s circuits—and their function.
In the past decade, the field received a welcome boost from new single-cell transcriptomic technologies, which identify neurons and other brain cells by measuring differences in patterns of gene expression. A family of techniques called spatial transcriptomics has taken this a crucial step further—telling researchers not only about a cell’s identity and function, but also how it fits into the brain’s elaborate wiring.
But spatial transcriptomics has had a nagging problem. Many different types of cells are often close neighbors in the brain, intermingling and rapidly sharing messages with one another. It means that current spatial transcriptomics methods don’t have good enough resolution to prevent mixing up genes from nearby cells and incorrectly identifying the cell types.
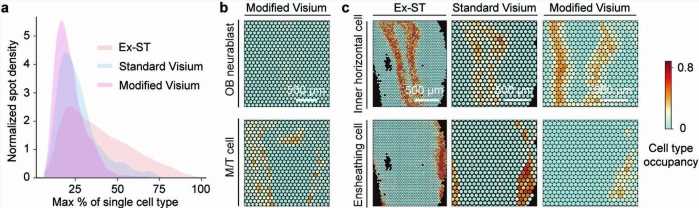
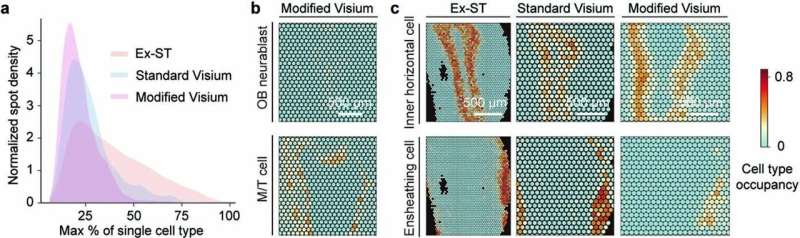
Now, in a paper published on June 22nd in Nature Methods, researchers at the Wu Tsai Neurosciences Institute at Stanford demonstrated a substantial improvement in the resolution of spatial transcriptomics using a crafty trick. By expanding the brain tissue so that it grew 2.5 times in size, Bo Wang, an assistant professor of bioengineering, and colleagues were able to successfully resolve neuron types in the mouse brain’s hippocampus and olfactory bulb in much finer detail than standard methods allow.
Wang is a member of the Neuro-omics Initiative—a Big Ideas in Neuroscience Initiative—which seeks to build new tools to improve our grasp of the brain at the molecular level of genes and proteins, and ultimately close the gap between our understanding of the microscopic molecules up to large-scale brain circuits and networks. The new technique, named expansion spatial transcriptomics (Ex-ST for short), is a prime example of a new tool that will propel the Neuro-omics Initiative forward in this mission.
An expanded view
Spatial transcriptomics relies on capturing genetic instructions called mRNA from tissue sections. These mRNA instructions are what guide our cells to make their protein machinery, and capturing them allows researchers to classify each cell in a larger tissue specimen. The problem was that the standard capture arrays weren’t dense enough to catch mRNA from tiny individual cells. Instead, each “spot” on the array captures mRNA from overlapping cell types, making it difficult to identify individual cells.
To get around the problem, Wang and his colleagues took a somewhat counter-intuitive approach. Rather than continue the decades-long journey neuroscientists have undertaken to improve the resolution of imaging tools, they took inspiration from the expansion microscopy technique pioneered by Ed Boyden’s lab at MIT. Boyden’s method expands the brain tissue itself so that it can be viewed at a higher resolution under a microscope.
Wang’s team decided to see if spatial transcriptomics could benefit from the same clever tissue-expanding trick. More specifically, they reasoned that if they could expand the cells, then each spot could capture mRNA from fewer cells, enabling greater resolution to identify different neurons.
To achieve the expansion, the research team—which included a collaboration with scientists at the Science for Life Laboratory in Stockholm, Sweden—developed a pipeline that starts with anchoring the mRNA to a gel matrix. Then, they apply enzymes that chew up all the proteins in the tissue that aren’t expandable until all that’s left is mRNA suspended in gel—what Wang calls a “ghost tissue.”
Here, the team benefited from another set of microscopy techniques developed by Stanford professor Karl Deisseroth. By anchoring tissue to a gel and then removing molecules that scatter light, these “clarity” techniques make brain tissue almost as clear as glass.
The “ghost tissue” in the gel expanded rapidly when placed in water (thanks to the effects of electrostatic repulsion). All in all, it swelled by about 2.5 times in all three dimensions.
But the impressive growth also led to the loss of mRNA, leaving minimal amounts to be captured. It was an incredibly challenging project, with two teams working across the globe during a pandemic.
“For a while, I thought, it’s never going to work. It’s like we hit a dead end,” said Wang. But Wang gives credit to the two graduate students who led the project—Yuhang Fan in his lab and Žaneta Andrusivová in Sweden—for persevering and eventually finding the tweaks that made the method work. “What I find amazing is that they were not discouraged by all the failures,” he said.
In the end, the arduous journey paid off. The researchers were able to validate that Ex-ST is better at resolving neuron types in the hippocampus and olfactory bulb. For example, looking at neurons in the CA1 region of the hippocampus, crucial to learning and memory, the increased resolution meant that they were able to distinguish the mRNA present in cells’ very long, antenna-like dendrites versus the more compact cell bodies. Thus, Ex-ST provides a new window into the gene activity happening at different places within single neurons.
Wang is hopeful that their new Ex-ST method will be immediately impactful to the thousands of neuroscientists around the world already using these methods. “They could benefit from our method because it’s only a small modification of their protocols, so they literally can just repeat their experiments and improve their results tremendously,” said Wang.
The future of the Ex-ST method
When Liqun Luo, professor of biology and a co-author on the paper, first saw the success of the Ex-ST method, he was excited that such a simple but clever idea turned out to be so effective. “It combines two cutting-edge technologies, expansion microscopy and spatial transcriptomics, to enable gene expression profiling in brain tissue with amazing—down to single-cell—resolution,” he said.
Luo is also a lead researcher on the Neuro-omics Initiative, and notes that he’s excited to now have the Ex-ST method on board. “A major component of Neuro-omics is to apply cutting-edge single-cell transcriptomic technologies to resolve neuron cell types,” said Luo. “Now, Ex-ST allows such analysis with much improved resolution, which lays an important foundation to understand the structure and function of brain tissue.”
Even with the vast improvements, the research team is still aiming to increase the resolution even further. Currently, they’re making progress towards developing a method that can decipher specific subtypes of mRNA, like those with certain modifications. It’s a task that only becomes possible because of their pioneering work to increase the efficiency of spatial transcriptomics.
In keeping with the goals of the Neuro-omics Initiative, Wang’s team is heading in this direction because it will reveal more about how the brain’s tiniest parts function. They’re not just using mRNA patterns to distinguish between different types of cells—they also want to know what these different genetic messages mean for cell function. “That’s where we’re going because we think that can give us more information in terms of what brain cells are really doing.”
More information:
Yuhang Fan et al, Expansion spatial transcriptomics, Nature Methods (2023). DOI: 10.1038/s41592-023-01911-1
Journal information:
Nature Methods
Source: Read Full Article