Blood cancer patients, especially when they relapse, have a poor prognosis, and generally do not survive very long. The last five years have seen success in treating these patients with Chimeric Antigen Receptor (CAR) T-cells—a type of immunotherapy where T-lymphocytes are collected from patients, genetically modified in the laboratory to express a ‘CAR’ and infused back into patients where they multiply and specifically target and kill cancer cells.
CAR T-cells have had remarkable success in treating several types of blood cancers, including B-acute lymphoblastic leukemia (B-ALL), large B-cell lymphoma and multiple myeloma. However, this type of treatment is logistically challenging to deliver and it can take several weeks for the CAR T-cells to be manufactured, during which time the cancer can grow to a stage where it becomes too difficult to treat.
An alternative to using CAR-T cells derived from cancer patients is to use pre-manufactured ‘off-the-shelf’ Chimeric Antigen Receptor (CAR) T-cells. ‘Off-the-shelf’ CAR T-cells are generated from healthy volunteer donors and undergo an additional ‘gene editing’ step to remove their own T-cell receptor, allowing them to be given to any patient with minimal delay and without causing graft versus host disease.
The CALM clinical trial, initiated at King’s in 2016, aimed to evaluate the therapeutic potential of UCART19—the first ‘off-the-shelf’ CAR-T cell product of its king against adult patients with B-ALL.
This multi-center trial was conducted in the UK, U.S. and Japan with much of the translational research carried out by the Cellular Immunotherapy Group at the Rayne Institute, School of Cancer & Pharmaceutical Sciences. The final results of this study were recently published in The Lancet Haematology and Cancer Research Communications, and build upon the preliminary data that were published in The Lancet in 2020.
A total of 25 adult patients with relapsed or treatment-resistant B-ALL who had no other treatment options were treated with chemotherapy drugs fludarabine and cyclophosphamide, with some additionally receiving an antibody called alemtuzumab. All patients were then given UCART19 ‘off-the-shelf’ CAR T-cells and followed up for adverse events and disease response.
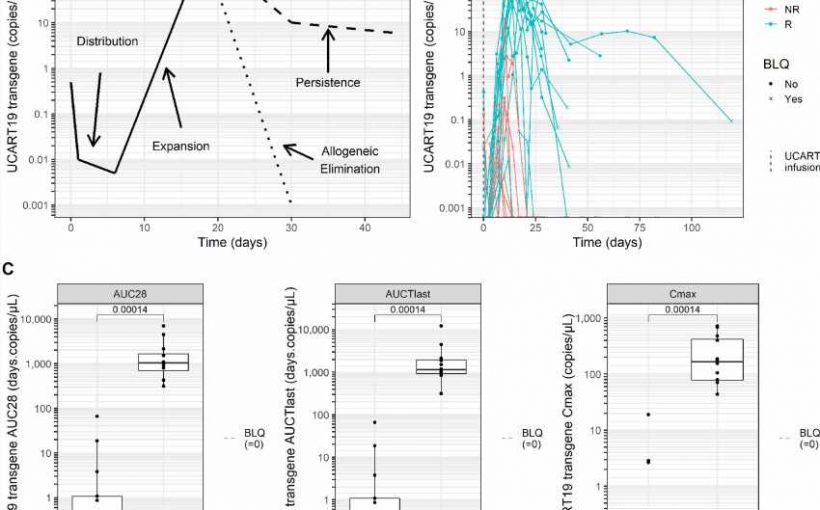
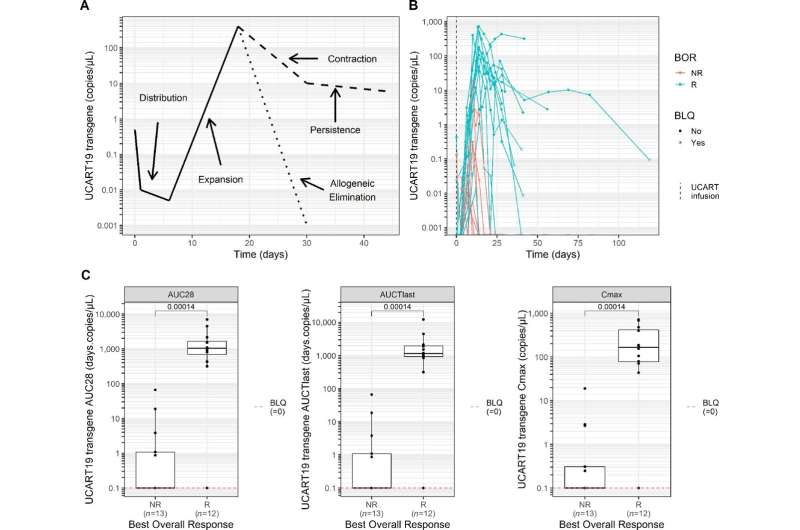
Side effects of the treatment were found to be manageable with no unexpected complications. 48% of treated patients achieved a complete remission lasting on average 7.4 months. Patients who showed signs of disease response had higher levels of UCART19 expansion than non-responders. Further analysis also found that alemtuzumab treatment was required for UCART19 expansion and disease response.
The findings from these two publications clearly highlight the therapeutic potential of UCART19 and ‘off-the-shelf’ CAR T-cell products more generally.
“The CALM Trial results are very promising and demonstrate, for the first time, the safety and feasibility of allogeneic ‘off-the-shelf’ CAR-T cells. Further work is underway to improve the persistence and long-term efficacy of these CARs,” says Dr. Reuben Benjamin, Clinical Senior Lecturer at KCL and Chief Investigator for the CALM study.
More information:
Reuben Benjamin et al, UCART19, a first-in-class allogeneic anti-CD19 chimeric antigen receptor T-cell therapy for adults with relapsed or refractory B-cell acute lymphoblastic leukaemia (CALM): a phase 1, dose-escalation trial, The Lancet Haematology (2022). DOI: 10.1016/S2352-3026(22)00245-9
Sandra Dupouy et al, Clinical Pharmacology and Determinants of Response to UCART19, an Allogeneic Anti-CD19 CAR-T Cell Product, in Adult B-cell Acute Lymphoblastic Leukemia, Cancer Research Communications (2022). DOI: 10.1158/2767-9764.CRC-22-0175
Journal information:
The Lancet Haematology
,
The Lancet
Source: Read Full Article