In a recent study posted to the medRxiv* preprint server, researchers analyze the effect of prior infection, vaccination, and hybrid immunity against the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) BA.1 and BA.2 Omicron subvariant infections. To this end, no discernible differences in protection profiles were observed in each group.
The team also found that the vaccination enhanced the protection of those with prior infection. As a result, the hybrid immunity resulting from prior infection and a recent booster vaccination (third dose) conferred the strongest protection against infection from either subvariant of Omicron.

Study: Effect of prior infection, vaccination, and hybrid immunity against symptomatic BA.1 and BA.2 Omicron infections and severe COVID-19 in Qatar. Image Credit: Niphon Subsri / Shutterstock.com
Difference in protection against BA.1 and BA.2 Omicron subvariants
The SARS-CoV-2 Omicron wave, which was initially dominated by the BA.1 subvariant and later by BA.2, started on December 19, 2021, and peaked in mid-January 2022 in Qatar. Although classified as subvariants of Omicron, both BA.1 and BA.2 are considerably genetically distant from each other, with not much reported on the protection of prior immunity against these subvariants.
Thus, the researchers of the current study aimed to investigate the effectiveness of protection granted by either prior SARS-CoV-2 infection, vaccination from either coronavirus disease 2019 (COVID-19) messenger ribonucleic acid (mRNA) vaccines from Pfizer-BioNTech (BNT162b2) and Moderna (mRNA-1273), or hybrid immunity generated from both prior infection and vaccination, against symptomatic BA.1 and BA.2 Omicron infections. Protection was also analyzed against the hospitalizations and deaths that were due to BA.1 and BA.2 subvariants.
Effectiveness of prior immunity against BA.1
While prior infection provided 50.2% effectiveness against symptomatic BA.1 infection, the primary vaccination of two-dose BNT162b2 vaccination provided negligible protection at -4.9%. Hybrid immunity gained from prior infection and the two-dose vaccination provided protection at effectiveness similar to that of only prior infection at 51.7%.
The effectiveness of a three-dose vaccination regimen was observed at 59.6%, whereas the effectiveness of hybrid immunity of prior infection and a three-dose vaccination was highest at 74.4%.
Concurrently, prior infection, vaccination, and hybrid immunity all demonstrated strong effectiveness of over 90% against severe, critical, or fatal COVID-19 due to BA.1 infection.
Effectiveness of prior immunity against BA.2
Similarly, the effectiveness of prior infection and two-dose BNT162b2 vaccination against symptomatic BA.2 infection was observed at 46.1% and-1.1%, respectively. Comparatively, hybrid immunity from prior infection and two-dose vaccination was observed at 55.1%, which was similar to the effectiveness of only prior infection.
Three-dose vaccination provided protection at 52.2% effectiveness, whereas hybrid immunity from prior infection and three-dose vaccination provided the highest effectiveness of protection at 77.3%. Prior infection, vaccination, and hybrid immunity all showed strong effectiveness exceeding 70% against hospitalizations and deaths due to the BA.2 subvariant.
The researchers also found that prior infection, vaccination, and hybrid immunity in the mRNA-1273-vaccine study showed similar effectiveness profiles against BA.1 and BA.2 to those in the BNT162b2-vaccine study.
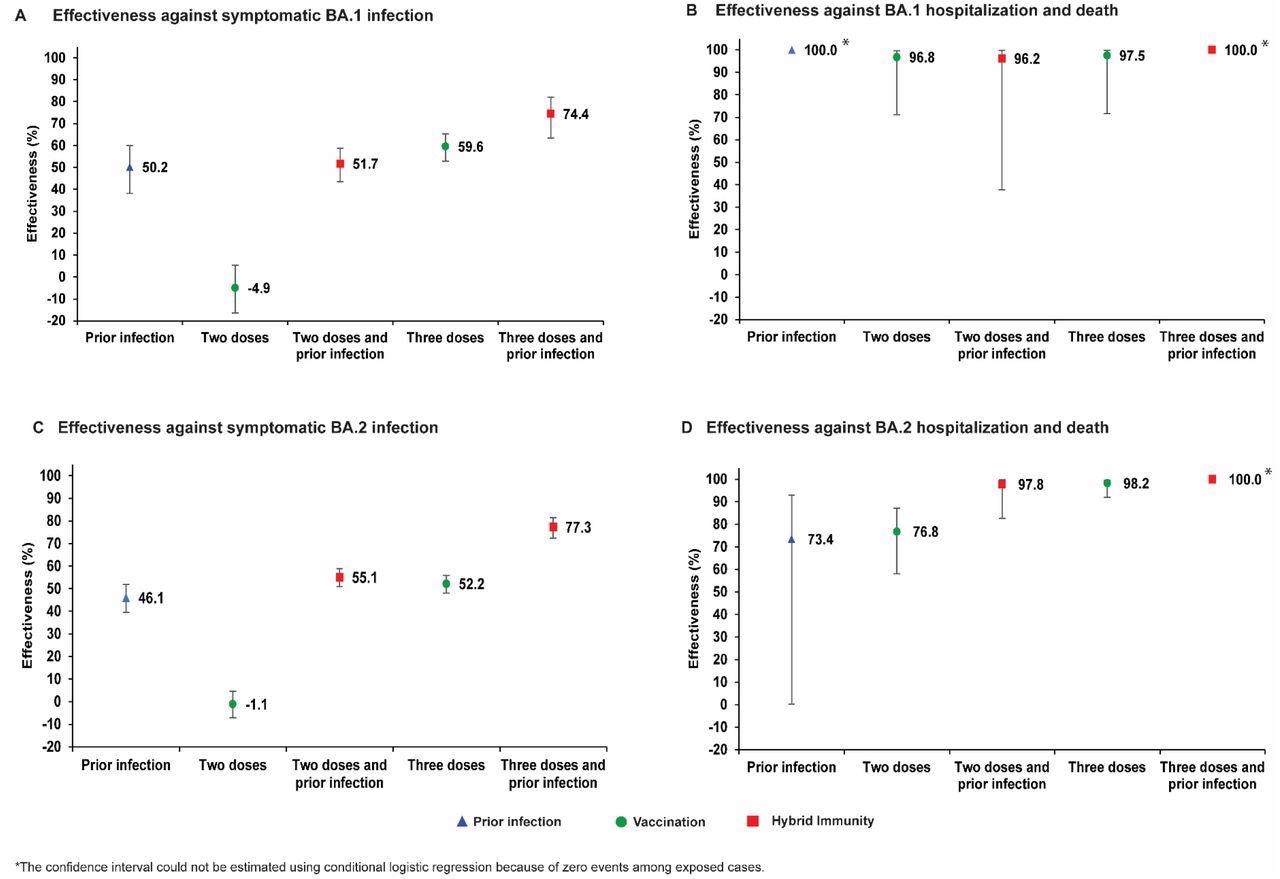
Effectiveness of prior infection, vaccination, and hybrid immunity against symptomatic Omicron infection and against severe, critical, or fatal COVID-19 for the BA.1 (panels A and B, respectively) and BA.2 (panels C and D, respectively) subvariants in the BNT162b2-vaccine study.
Conclusions
Prior infection with a pre-Omicron variant nearly a year earlier was associated with about a 50% reduced risk of infection. There were no discernable differences in protection of prior infection against either BA.1 or BA.2.
A two-dose primary vaccination series had negligible effectiveness against either BA.1 or BA.2. However, the booster dose was associated with about 60% reduced risk of infection. There were no discernable differences in the protection of booster vaccination against either BA.1 or BA.2.
Based on the finding that protection of hybrid immunity of prior infection and primary-series vaccination was similar to that of prior infection alone at about 50%, the researchers suggest that this protection originated from prior infection, rather than from primary-series vaccination. The highest protection was conferred by hybrid immunity of prior infection and recent booster vaccination at about 80%.
Although the different forms of immunity showed large differences in protection efficacies against symptomatic infection, they all showed strong protection against COVID-19 hospitalization and deaths.
“This suggests that any form of prior immunity, whether induced by prior infection or vaccination, is associated with strong and durable protection against COVID-19 hospitalization and death.”
*Important notice
medRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.
- Altarawneh, H. N., Chemaitelly, H., Ayoub, H. H., et al. (2022). Effect of prior infection, vaccination, and hybrid immunity against symptomatic BA.1 and BA.2 Omicron infections and severe COVID-19 in Qatar. medRxiv. doi:10.1101/2022.03.22.22272745, https://www.medrxiv.org/content/10.1101/2022.03.22.22272745v1
Posted in: Medical Research News | Disease/Infection News | Pharmaceutical News
Tags: Coronavirus, Coronavirus Disease COVID-19, immunity, Omicron, Respiratory, Ribonucleic Acid, SARS-CoV-2, Severe Acute Respiratory, Severe Acute Respiratory Syndrome, Syndrome, Vaccine

Written by
Namita Mitra
After earning a bachelor’s degree in Veterinary Sciences and Animal Health (BVSc) in 2013, Namita went on to pursue a Master of Veterinary Microbiology from GADVASU, India. Her Master’s research on the molecular and histopathological diagnosis of avian oncogenic viruses in poultry brought her two national awards. In 2013, she was conferred a doctoral degree in Animal Biotechnology that concluded with her research findings on expression profiling of apoptosis-associated genes in canine mammary tumors. Right after her graduation, Namita worked as Assistant Professor of Animal Biotechnology and taught the courses of Animal Cell Culture, Animal Genetic Engineering, and Molecular Immunology.
Source: Read Full Article
