The coronavirus SARS-Cov-2 can cause severe organ damage in humans. Heart complications are one of the possible consequences of an infection. In addition, the virus also attacks the heart directly, can cause myocarditis and lead to heart failure. Dr. Nazha Hamdani, head of the research department for molecular and experimental cardiology at the Bochum university hospital, has tracked the journey of the virus in detail.
She and her group have found that SARS-Cov-2 infects human cardiac muscle cells, and that this infection is primarily fueled by inflammation and oxidative stress. This occurs mainly in patients with comorbidities such as obesity, diabetes and hypertension. The International Journal of Cardiology has published an article on these findings.
Virus detected in heart cells
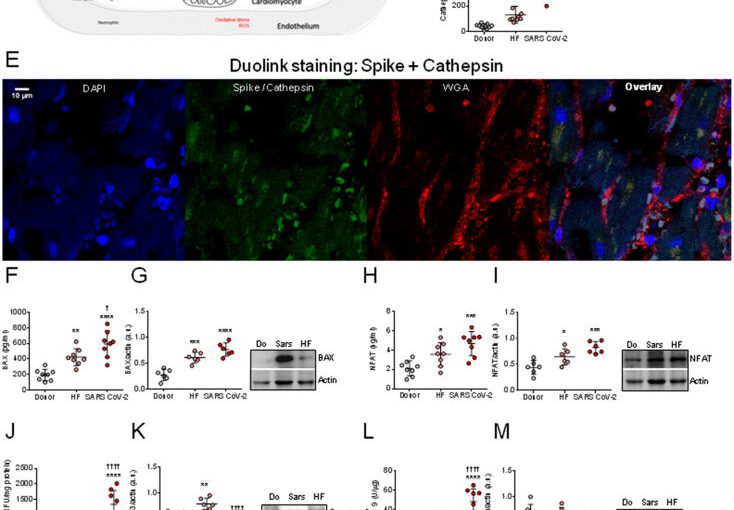
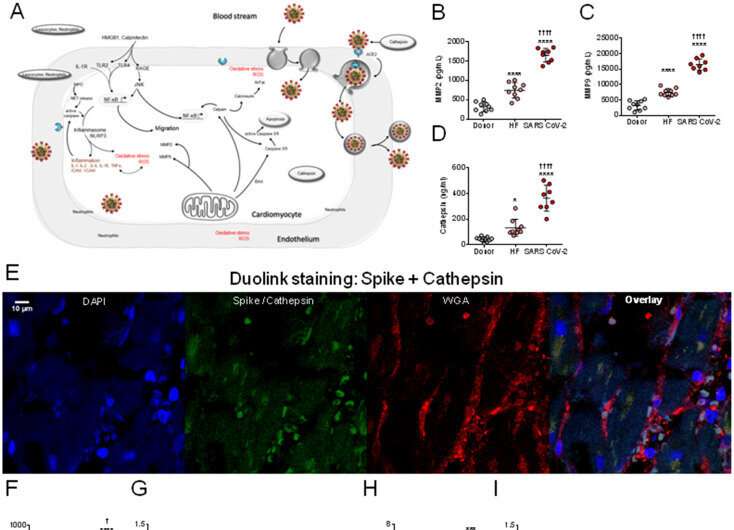
In order to track down the new entry mechanism, the research team at the university hospital used histochemical methods and microscopy to analyze heart tissue structures from patients suffering from COVID-19 and those who died from or with the disease. In a first step, they provided evidence that the virus can indeed be detected directly in the cells of the heart muscle. “Our observations show that the virus exerts pressure on the heart muscle, attacks and weakens the contractile force, i.e., the pumping function of the heart,” says Hamdani.
But how does the virus enter the heart? What mechanisms facilitate the penetration of the virus into the heart muscle cells? The Bochum team showed that one possible mechanism of cardiac muscle cell dysfunction in SARS-Cov-2 patients is the activation of certain enzymes that degrade proteins. In fact, the team detected an increased so-called proteolytic activity.
This suggests that SARS-Cov-2 enters cells as a result of the activation of the spike protein by enzymes responsible for the degradation of proteins, and that its entry into cells depends on these degradation enzymes. In addition, Hamdani’s group investigated proteins that are responsible for apoptosis, i.e. cellular suicide. The team showed that while the apoptotic proteins had increased activity, their expression was drastically reduced. “This indicates that the proteins are cleaved and apoptosis is activated,” explains Nazha Hamdani. “The results imply that apoptosis contributes to the deterioration in cardiac contractility observed in SARS-Cov-2 patients.”
The key role of inflammation and oxidative stress
In the next step, the team set out to explore what promotes the increased proteolytic activity and apoptosis of cardiac myocytes. The study showed that oxidative stress and a pro-inflammatory environment exacerbate the damage associated with SARS-Cov-2. The focus here was on the so-called neutrophils. Neutrophils are one of the primary cell types that release proteolytic enzymes. They play an essential role during an inflammatory response. They are rapidly mobilized from the bloodstream into the damaged tissue. Since proteolytic enzymes are released more frequently in SARS-Cov-2 patients, Hamdani’s team analyzed the signaling pathway, more specifically the interleukin-6-driven neutrophil traffic. The researchers found that inflammatory signaling pathways in cardiac myocytes were highly regulated—i.e., interleukin-6 was highly elevated—suggesting a key role for these white blood cells in COVID-19 and associated inflammatory pathologies.
Alternative gateways
Furthermore, the Bochum-based researchers have backed the existing findings that the virus also uses the protein neuropilin-1 (NRP-1) as a gateway into the cells. Hamdani’s research shows that the coronavirus thus has several mechanisms at its disposal to spread in human organs.
Source: Read Full Article