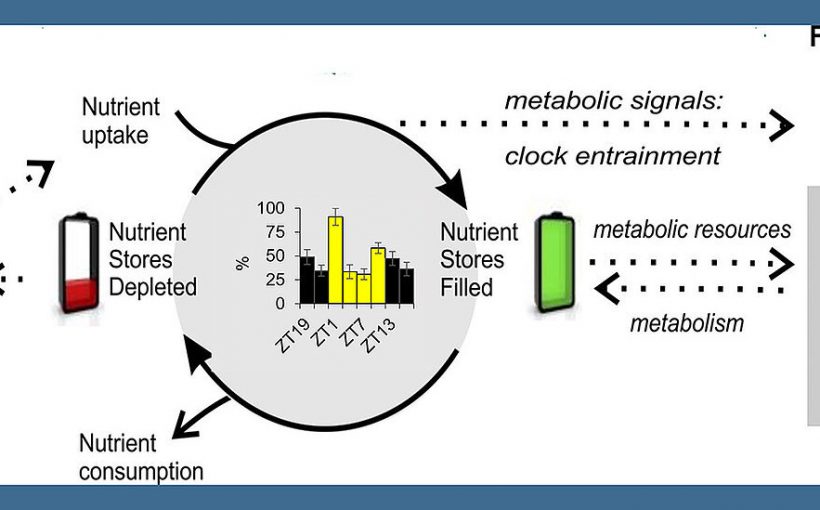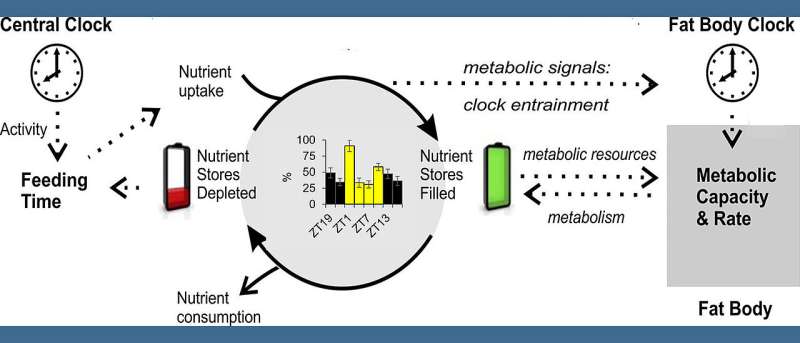
In the fruit fly Drosophila, circadian clocks also control fat metabolism. This is shown in a new study by a research team at the University of Würzburg. The findings could also be relevant for humans.
Much is known about how modern human lifestyles contribute to triggering metabolic disorders and diseases. Irregular meals, eating late in the evening or at night, and lack of prolonged periods of abstinence are now considered major factors in the development of the so-called “metabolic syndrome”—a clinical picture characterized, among other things, by obesity and high blood pressure, elevated blood sugar, and impaired lipid metabolism. However, the underlying mechanisms are still poorly understood.
Deepening knowledge of disturbed metabolism
A new study by scientists at the Biocenter of the Julius-Maximilians-Universität Würzburg (JMU) could now help to deepen our knowledge of the disturbed metabolic processes.
The study led by Professor Christian Wegener from the Department of Neurobiology and Genetics at JMU and Dr. Agnes Fekete, manager of the Metabolomics Core Unit at the Department of Pharmaceutical Biology, has now been published in the Journal of Lipid Research. In this work, the team investigated the extent to which internal clocks control lipid metabolism in the fruit fly Drosophila.
“Lipids, or fats, are macro-nutrients that serve in the organism, for example, as building blocks for biological membranes, as signaling substances and as long-term energy stores. In order to reach their target cells, lipids must be transported via the bloodstream after ingestion from the intestine or when mobilized from fat stores,” says Agnes Fekete, describing the background to the study.
However, this “cycle of lipids” can be disturbed by modern lifestyles as shift work, irregular mealtimes and the permanent availability of food do not correspond to the rhythm dictated by the day-night cycle and accordingly entrained internal clocks.
An internal clock tunes lipid transport to the siesta
The team led by Wegener and Fekete therefore used the fruit fly as an example to investigate the regular fluctuations in lipid metabolism in this insect. They tested the role of internal clocks in this process and how factors such as light, food intake and food composition affect it. Their special focus was on the so-called hemolymph—the analog to the human bloodstream—and the lipid molecules transported in it.
Wegener states, “Our data indicate that the circadian clock coordinates the daily oscillations of the transport lipids in the hemolymph with the resting periods of the fruit fly, the so-called anabolic siesta phase. However, the shape and strength of these oscillations is strongly influenced by light,” said the neurobiologist.
Both normal, healthy fruit flies and mutants in which the internal clock was genetically switched off were used in this study. The flies were kept under different light conditions—sometimes in a regular light-dark rhythm, sometimes in constant darkness. In addition, they were given different “diets”—from a pure sugar medium to the standard medium, which contained all nutrients in abundant quantities.
Clear indication of an oscillation controlled by internal clocks
Via the hemolymph, the team regularly monitored the concentration in which the fly transported certain lipids through its body. Clear patterns emerged: In “healthy” flies, for example, kept in a light-dark cycle on pure sugar medium, the concentration of lipids was high especially at the beginning and the end of the light phase. Under constant dark conditions, only one increase was found, always in the middle of the “subjective” day. Flies without a functioning circadian clock lacked such regular changes.
These changes were also less pronounced when the flies were fed a full diet rather than just sugar. In this case, the rhythmic changes in hemolymph lipid levels were greatly attenuated. The fact that lipid concentrations peaked once in the middle of the light phase during a time-limited feeding, regardless of the time of food intake, is seen by the scientists as a “clear indication of an oscillation controlled by internal clocks.”
Starting point for a better understanding in humans
Of course, these processes in Drosophila differ in some aspects from those in humans or other mammals. Nevertheless, the team led by Wegener and Fekete is convinced that their work can serve as a starting point to in detail dissect the underlying general mechanisms using the genetic tools available for Drosophila—and thus, in the longer term, also provide a starting point for understanding similar processes and their pathological changes in humans.
In a next step, the scientists now want to investigate the role of the circadian clock in the intestine and in the fat body—analogous to liver and fat tissue in humans—for rhythmicity. To do this, they want to genetically switch off only these so-called peripheral clocks or desynchronize them with respect to the other body clocks. “This would be an animal model that could be used to study the effects of desynchronizing the body clocks, for example, by regular food uptake during the night,” says Christian Wegener.
More information:
Kelechi M. Amatobi et al, The circadian clock is required for rhythmic lipid transport in Drosophila in interaction with diet and photic condition, Journal of Lipid Research (2023). DOI: 10.1016/j.jlr.2023.100417
Journal information:
Journal of Lipid Research
Source: Read Full Article