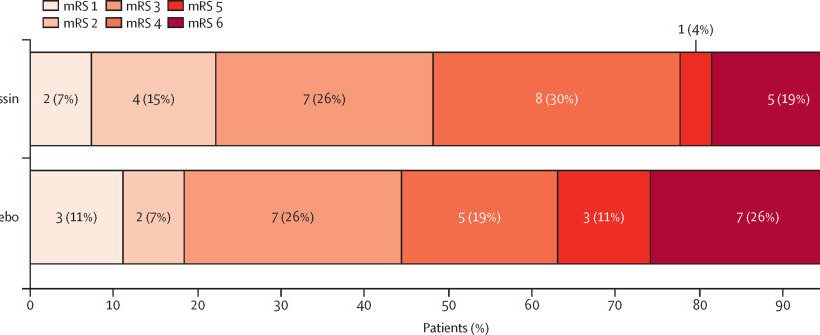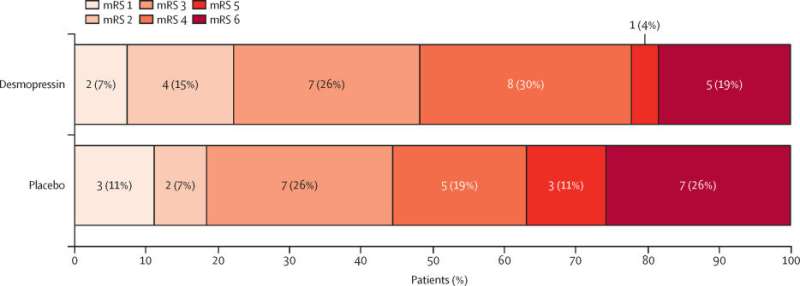
The results of a clinical trial have shown that a drug commonly used for patients with bleeding disorders has the potential to be used to lessen the side effects of blood-thinning drugs for patients who have experienced a stroke.
Researchers from the University of Nottingham and Oxford University Hospitals NHS Foundation Trust assessed the suitability of Desmopressin to be used in larger trials to help reduce the number of people who die or are disabled after intracerebral hemorrhage. The results from the DASH trial have been published in The Lancet Neurology.
Approximately 3 million deaths each year are due to spontaneous intracerebral hemorrhage worldwide and there is currently no proven effective drug treatment. Researchers estimate that two-thirds of survivors are left dependent on others and a quarter of patients were taking antiplatelet drugs at the time of incident.
Patients from 10 hospitals across the U.K. who had suffered an intracerebral hemorrhage while taking antiplatelet drugs took part in the clinical trial. One group were given Desmopressin while a second group was given a “dummy drug.”
Dr. Michael Desborough, Department of Clinical Hematology, Oxford University Hospitals NHS Foundation Trust, said, “Intracerebral hemorrhage for people taking antiplatelet drugs leads to thousands of deaths every year. Unfortunately, at present there are no treatments available. The results of the DASH trial are an important step towards assessing whether desmopressin might reduce the high risk of death or disability from intracerebral hemorrhage worldwide.”
Professor Nikola Sprigg, Stroke Trials Unit, University of Nottingham, added, “Whether Desmopressin reduces the number of people who die or are disabled after intracerebral hemorrhage is an important question to answer. These findings support the need for a definitive, large-scale trial to determine if desmopressin improves outcomes in patients with intracerebral hemorrhage on antiplatelet drug therapy.”
More information:
Michael J R Desborough et al, Desmopressin for patients with spontaneous intracerebral haemorrhage taking antiplatelet drugs (DASH): a UK-based, phase 2, randomised, placebo-controlled, multicentre feasibility trial, The Lancet Neurology (2023). DOI: 10.1016/S1474-4422(23)00157-6
Journal information:
Lancet Neurology
Source: Read Full Article