
Huge amounts of time and money have been devoted to finding treatments for diseases that become more common as we age, like cancer and Alzheimer’s. However, Leonid Peshkin, a lecturer in systems biology in the Blavatnik Institute at Harvard Medical School, is among a growing number of scientists who view such diseases as symptoms of a bigger and more universal process: Aging itself.
“I’ve always felt like aging is a disease that is no different than any other disease, and just because we are so used to it, we shouldn’t take it for granted,” Peshkin said. “I want to look at the root cause of aging and understand it fundamentally. What is the cause and what is the effect? What is high up in this cascade? And most importantly: How can we fix aging?”
Despite earning a Ph.D. in machine learning and artificial intelligence, Peshkin has always been fascinated by the concept of aging, so when he had the chance to pivot into aging research, he took it. Now, Peshkin is combining his expertise in computer science with skills he has gained in biology to develop a new model system for aging research. He hopes that his research will illuminate fundamental features of aging and ways to address them as well as encourage scientists with other skills to get involved.
Peshkin spoke to Harvard Medicine News about how he plans to use his new model system to study aging and why he thinks crowdsourcing is the key to advancing the field more quickly.
What are some of the problems in how people are currently studying aging?
In the field of aging, a naive view is that people are searching for the elixir of life—a supplement or a pill that extends life. There is a vast body of literature where people claim that certain drugs, diets, or regimens extend the lives of model organisms such as ants, worms, flies, fish, or mice. People do an intervention, measure how long the animals live, get an extension of median life of 10, 15, or 20 percent, and publish a paper. There are several problems with this approach.
One problem is that papers—even those on the same species—often use different controls, making it impossible to compare results. We’re lacking nice, standardized data about life span across laboratories and across organisms. Additionally, studies often use mice that are engineered to age rapidly, living for only a couple of months. Extending the life span of these mice from two to three months looks like a huge accomplishment, but probably has very little to do with extending healthy life span. Which leads to another set of questions: What are we trying to do? Are we extending life span for the sake of life span? We don’t want an organism to live longer if it has a miserable, demented, frail existence. We want to see how much an animal eats, how it procreates, how well it reacts to stimuli. Health span, not life span, is key.
How does your new model system address these problems?
My colleagues and I realized that we need a standardized, scalable system we can use to test how drugs, diets, and other interventions affect behavior, reaction to stimuli, and additional measures of health span. We started developing a system using Daphnia magna, a species of water flea that has been used in toxicology and environmental research for decades, but hasn’t been used to study aging.
What’s so great about Daphnia? The species has a life span of one month, and even though it’s an invertebrate, it is a complex organism. It is beautifully transparent, with a beating, two-chambered heart, an innate immune system, eyes, a brain, and muscle tissue. In fact, when we use electron microscopy to zoom in on the cells of Daphnia, we see that the neurons and muscle cells look very similar to human neurons and muscle cells. Daphnia is also extremely sensitive to small concentrations of drugs. Finally, Daphnia is parthenogenetic, or clonal, so the offspring are identical.
Our system, which was developed jointly by aquaculture specialist Rachael Jonas-Closs and engineer Yongmin Cho at HMS, consists of a fridge-sized incubator with many one-liter Daphnia tanks inside. The tanks are flat, so the animals mostly move in two dimensions. A camera records a one-minute video once per day for 30 days. We collect these videos and analyze them to quantify many motion features of the animals, such as how much they are reacting to light and how long their jumps are. All of this behavior is characteristic of age—much like humans, you can predict the age of Daphnia based on how they move. We hope to identify interventions that don’t necessarily extend the life span of Daphnia, but do extend active life and health.
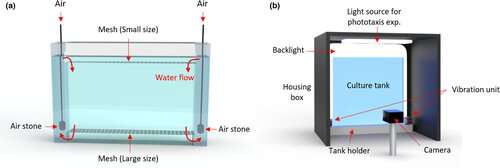
You recently published a paper in Aging Cell on your Daphnia system. What did you show in this study?
This paper is establishing the baseline for Daphnia as a new model organism for studying aging. We describe the system in detail, including how we set up the tank, fed the animals, removed new offspring, and set the light cycles and temperature. These appear to be boring details, but the whole point is getting the boring details right. We are developing a set of routines that are needed to raise Daphnia in a standardized way that is also scalable.
We also demonstrated the potential of our system for studying aging. When you turn on a light, Daphnia has a reflex to move towards it, and when you turn off the light, they hide. It’s a very clear response. In our paper, we show that old Daphnia ignore the light and middle-aged Daphnia have a mixed response: some react, some don’t, and some react slowly. This is a nice behavioral assay where our one-minute videos can capture how the reaction to light diminishes with age.
We decided to test metformin, a common diabetes drug that, in the literature, has been shown to extend the life span of worms and flies. We got a beautiful negative result—we conclusively showed that metformin does not affect the life span of Daphnia. We did not necessarily prove or disprove the other papers, but we demonstrated that our system can be used to test a drug of interest and get solid, statistically significant results from a large sample. We also made Daphnia “drunk” by adding ethanol to the tank. Adding 3 percent ethanol had a strong effect on Daphnia behavior, providing proof of concept that our platform can detect the effects of various drugs and interventions.
Your Daphnia system is part of what you call ‘radically open, Wikipedia-style science.’ What is this concept?
Many people are passionate about aging research. Students ask for projects, volunteers from all over the world write to ask what they can do. There is this huge enthusiasm. These people aren’t necessarily experts on aging, but they are hackers, data analysis experts, zoologists, engineers, computer scientists, or even biologists from other disciplines. In building this Daphnia system, I realized that we are very nicely and crisply formulating some bite-sized problems of what has to be done that others can then address. With all of these people willing to help, there is an opportunity to crowdsource some of the science. I began to think about how can we use crowdsourcing to make science more efficient.
Our Daphnia platform is standardized, and consists of a tank that is easy to assemble and animals that are easy to maintain. It is great for educational purposes because people can play and observe. Anyone can do experiments in their lab, no matter where they are in the world. You could do experiments in your basement, test interventions, and immediately upload your one-minute video recordings to a server. With our system, many people can do science, and put the measurements online as soon as they are collected. Some of the measurements will be garbage, but that’s how Wikipedia works—it is organized in a way where it’s self-correcting. People are going to do crazy stuff with the Daphnia system, but if three teams in three different places repeat the same experiment, it will self-correct.
The idea is that people can assemble the Daphnia system and use it in their own experiments, and they can also improve the system by designing better tanks or developing better machine learning tools to analyze the videos. My job is to develop a cheap, scalable, and reproducible system for Daphnia, and I hope that the system will eventually take off and have a life of its own.
What do you hope to accomplish with your system?
We hope to both screen new drugs and verify drugs reported from research in other organisms. We do not think that any of these drugs are going to substantially extend the life or health of Daphnia. We expect some of them will extend life or health just a little bit. That’s why we need thousands of animals and dozens of tanks per experiment: we need a big sample size to reliably find these small differences. Once we find drugs that reliably statistically significantly extend health span a little bit, that gives us the opportunity to ask what are those drugs, what are the targets of those drugs. Then we will know how we should further focus our search.
Source: Read Full Article
