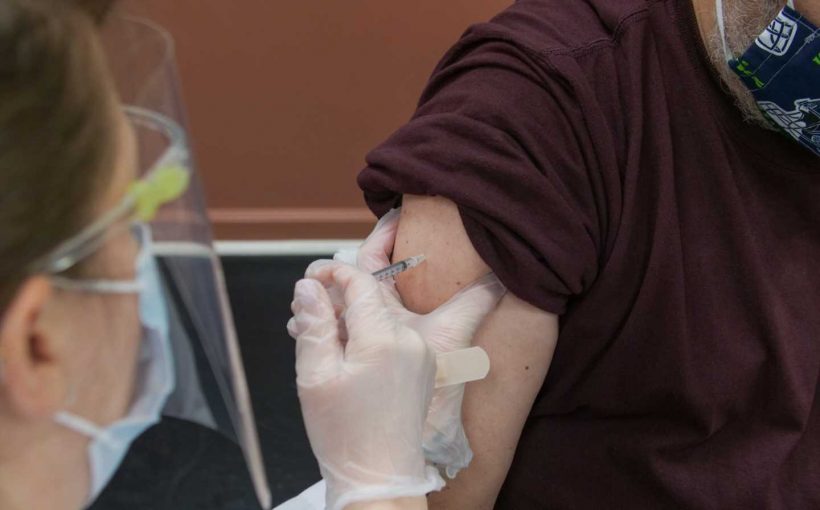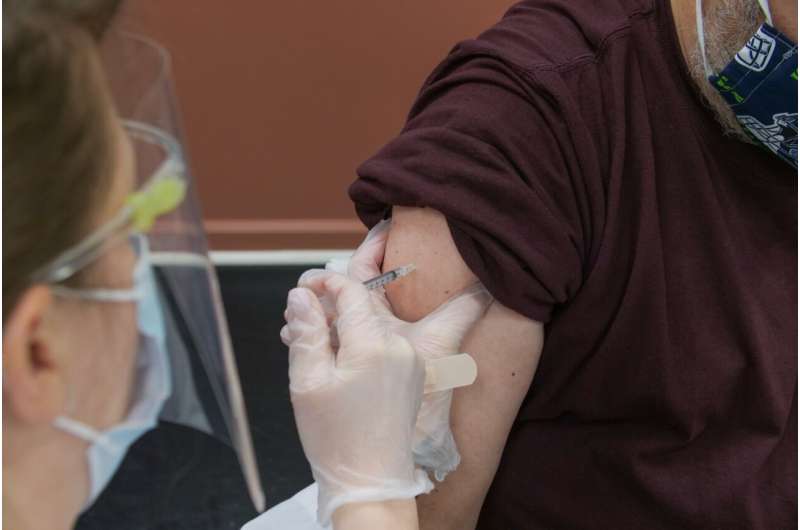
Canada on Tuesday approved an updated Moderna COVID vaccine targeting new variants, and is reviewing others likely to be rolled out this fall to mitigate infections once more on the rise.
This comes after the United States on Monday approved Moderna and Pfizer vaccines that correspond to an Omicron sublineage.
“I know we all wish COVID-19 no longer existed,” Health Canada’s chief medical advisor Supriya Sharma told a news conference in Ottawa.
“But people are still getting infected and vaccination continues to be one of the most effective ways to protect ourselves against serious outcomes including severe illness, hospitalization and death,” she said.
“Receiving a shot of the new formulation will help protect people against the variants circulating currently and expected to circulate through the fall and winter.”
The updated Moderna vaccine specifically targets the Omicron XBB.1.5 subvariant and has been approved for people six months of age and older.
Sharma said Pfizer-BioNTech’s revised vaccine, as well as a proposal for an updated shot from Novavax are also “being reviewed on a priority basis.”
Most Canadians have had at least two doses of a COVID vaccine, but the protection wanes over time. Only a small number have gotten boosters so far this year.
After a bad 2022-2023 respiratory virus season that saw a combination of influenza, COVID and RSV (respiratory syncytial virus) cases overwhelm hospitals, officials said they are now hoping to see more people roll up their sleeves for flu and COVID shots in the coming months.
© 2023 AFP
Source: Read Full Article