Introduction
What changes occur in the heart following myocardial infarction?
In vivo preclinical models of myocardial infarction
In vitro and ex vivo preclinical models of myocardial infarction
References
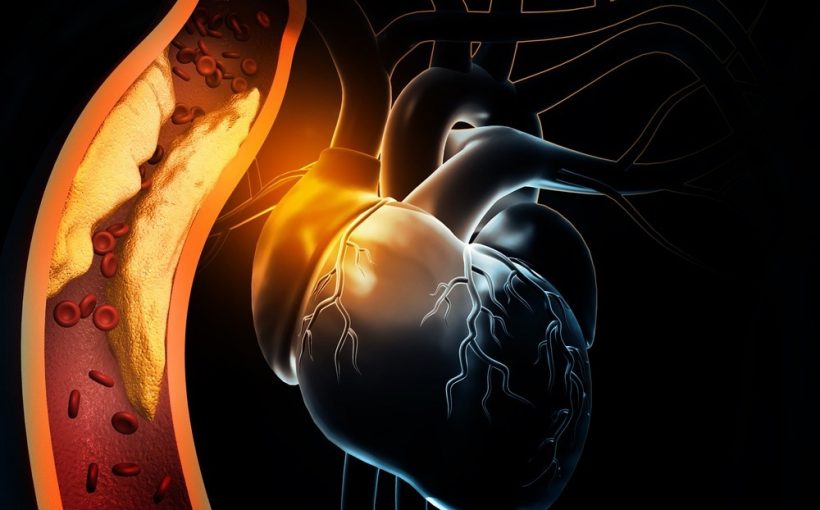
Myocardial infarction (MI) is permanent damage to the heart following myocardial ischemia, where blood flow to the heart muscles is blocked. Those with MI are at significantly higher risk of further cardiac issues for their remaining life, and cardiovascular diseases remain the leading cause of death.
In the early stages of drug development, safety, and efficacy must be established in non-human subjects, and in vivo rodent models of MI have been critical to the development of many such drugs now used regularly, such as ACE inhibitors. Unfortunately, non-human models often do not transfer well to the clinic, either because the drug is in some way ineffective or dangerous to humans specifically or because the preclinical model of MI used to establish efficacy was not suitably reflective of the condition in humans. In light of this, several preclinical models are utilized in cardiovascular research that aim to accurately model MI symptoms as closely as possible and will be discussed below.
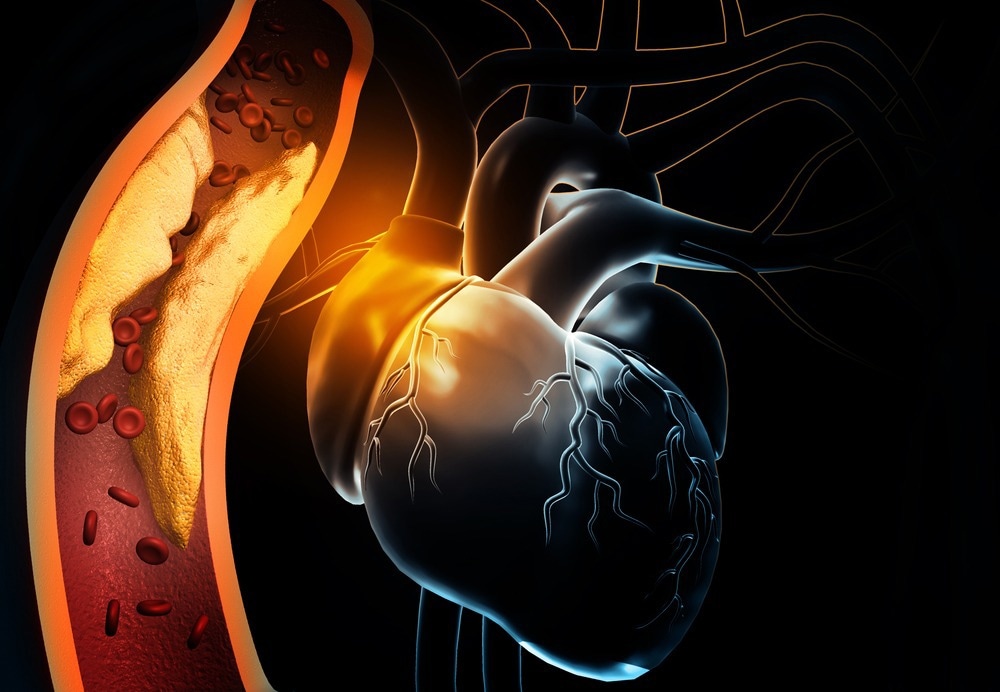
Image Credit: Explode/Shutterstock.com
What changes occur in the heart following myocardial infarction?
During myocardial ischemia, the heart muscles are starved of oxygen and quickly die, creating a distinct border between the infarct zone and the surrounding healthy tissue. Chemokine releases signals for macrophages to the region that begins to phagocytose dead cells and clear the area to allow regrowth, and extracellular matrix remodeling starts.
In adults, cardiomyocyte cells cannot proliferate completely to regain lost tissue, and thus a collagen scar is deposited by cardiac fibroblasts to maintain integrity. The resulting disorganized heart musculature often leads to ventricular arrhythmias as ordinary electrical signaling and controlled contraction is lost.
Physically, this leads to the weakening of the left ventricle wall and increased muscular stiffness, resulting in impaired contractility and lower ejection volume. Subsequently, in an attempt to compensate for reduced contractility, hormonal and protein receptor changes occur to enhance the presence of intracellular Ca2+. To accurately model MI preclinically, all of these factors must be accounted for and represented in the most accurate manner possible, generally achieved in vivo.
In vivo preclinical models of myocardial infarction
Given their ubiquity and high turnover, rodents are frequently the first choice of in vivo models. Mice are extremely well characterized and well understood genetically, with various genetically modified strains to choose from for the best applicability. Unfortunately, the small size and high heart rate of mice, around 500 beats per minute, make surgery and imaging very difficult, and thus rats are often used in place. Similarly, the slightly larger size of rabbits makes them appealing secondary in vivo models, with the added advantage of better mimicking human cardiac electrophysiology than rats or mice.
One of the most common surgeries performed on rodents to generate preclinical MI models is artery ligation, where a suture is tied around the left anterior descending coronary artery. This prevents blood flow to the left ventricle and induces cell death and scar tissue formation, similar to MI. Unfortunately, given the extreme technical complexity involved in performing artery ligation on many test subjects, in addition to variation introduced depending on subject species, age, sex, etc., infarction sizes are often inconsistent. More uniform infarctions can be produced in rodents by cryoinjury or electrical damage, where a probe is placed in contact with the anterior left ventricle wall to induce highly localized cell death. Less cardiac remodeling and thicker scar formation are observed by cryoinjury than artery ligation, but otherwise, the technique mimics MI well.
Immunology eBook

Artery ligation and cryoinjury ablation have been used widely in rodents to investigate the cellular and molecular mechanisms following MI, allowing MI's local and systemic effects to be described and extrapolated to humans. However, in many cases, it is advantageous to utilize larger animals as preclinical MI models, for which pigs, sheep, and dogs are most commonly employed. These animals are physiologically and anatomically closer to humans regarding heart size, heart rate, blood pressure, and other relevant parameters such as Ca2+ ion channel expression.
MI is usually induced temporarily in large animal models without open chest surgery by the insertion of a balloon into the coronary artery that is inflated to prevent blood flow to the left ventricle for around an hour, mimicking a human undergoing myocardial ischemia and receiving medical treatment that allows reperfusion to the area. Another advantage of inducing MI in this manner is that infarctions are sub-endocardial, unlike the surface-level infarctions induced by cryoinjury in rodents, which more accurately reflects MI in humans. The larger size of dog, pig, or sheep hearts compared with rodents allows easier and more detailed monitoring and data collection.

Image Credit: AgriTech/Shutterstock.com
In vitro and ex vivo preclinical models of myocardial infarction
While in vivo models best replicate MI in humans and allow the mechanisms underpinning MI and new therapeutics to be explored, many confounding factors may make investigating a specific question difficult, and for this purpose, various ex vivo and in vitro models of MI have also been developed. Ex vivo and in vitro models remove the influence of the biological system and allow parameters to be set for investigation, improving reproducibility and reliability.
For in vitro testing, rodent heart cells are commonly cultured and exposed to hypoxic environments to simulate MI, whereupon the biochemistry taking place can be studied in detail. For ex vivo testing, the Langendorff technique is used to isolate whole hearts for study, most commonly from rodents and other small animals, though dog hearts have also been isolated and perfused in this manner.
In the Langendorff technique, the heart sits in a nutrient-rich oxygenated buffer while blood substitute is pumped into the aorta in a retrograde perfusion process. As the blood enters the heart via the aorta, the aortic valve is forced shut, pushing the oxygenated solution into the coronary vessels and supplying the heart cells with oxygen.
References:
- Martin, T. P., et al. (2021) Preclinical models of myocardial infarction: from mechanism to translation. British journal of pharmacology, 179(5). https://bpspubs.onlinelibrary.wiley.com/doi/10.1111/bph.15595
- Wang, D., et al. (2019) A Cryoinjury Model to Study Myocardial Infarction in the Mouse. Journal of visual experiments, 19(151). https://pubmed.ncbi.nlm.nih.gov/31609308/
- Bell, R. M., et al. (2011) Retrograde heart perfusion: the Langendorff technique of isolated heart perfusion. Journal of molecular cell cardiology, 50(6). https://pubmed.ncbi.nlm.nih.gov/21385587/
Further Reading
- All Myocardial Infarction Content
- Myocardial T1 and T2 Mapping
Last Updated: Feb 6, 2023

Written by
Michael Greenwood
Michael graduated from Manchester Metropolitan University with a B.Sc. in Chemistry in 2014, where he majored in organic, inorganic, physical and analytical chemistry. He is currently completing a Ph.D. on the design and production of gold nanoparticles able to act as multimodal anticancer agents, being both drug delivery platforms and radiation dose enhancers.
Source: Read Full Article