A large team of researchers at Emulate Inc., working with colleagues from several institutions in the U.S., has found that human liver-chips are showing promise as a predictive toxicology tool. In their paper published in the journal Communications Medicine, the group describes how they developed their Organ-on-a-Chip technology and how well it has worked when tested with a host of drugs.
As the researchers note, one of the most expensive parts of developing new drugs is high attrition rates. Many drugs make it through the development process, testing and trials only to be found problematic when given to human patients. The main reason for it, they note, is that so much of the development of drugs for humans involves testing using animals.
Because of this shortcoming, pharmaceutical companies have been looking for other ways to test new drugs. One such new way is the development of microfluidic chips that mimic the behavior of certain organs in the human body—such as the liver. Such chips are made by emulating the physical structure of certain parts of the target organ and then adding real human cells harvested from real human organs. In this new effort, the team at Emulate Inc. has created what it calls an organ-on-a-chip, which they have trademarked as “Organ-Chip.”
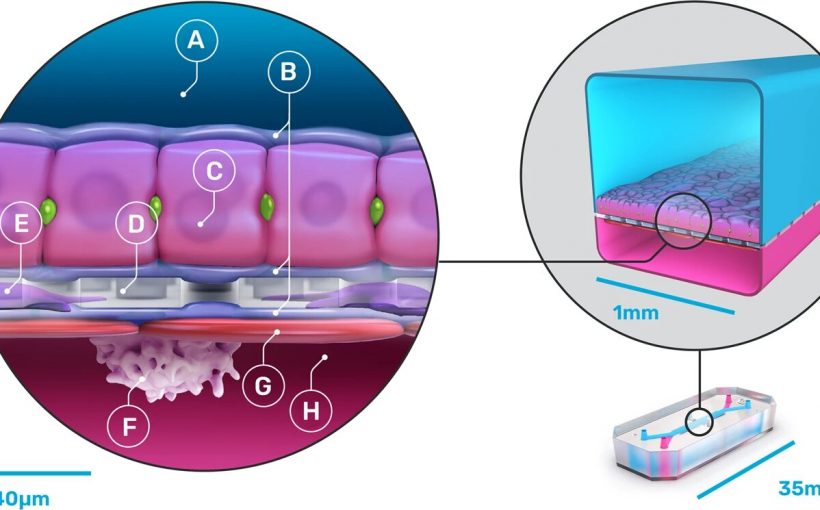
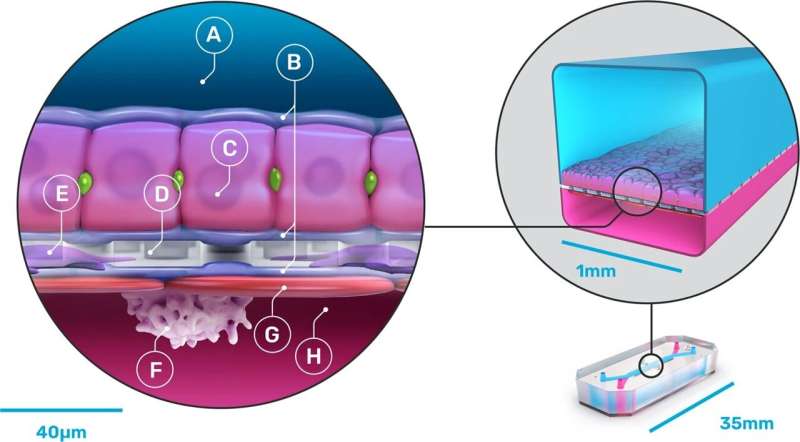
They are working on several such chips, but in this new effort, they focused exclusively on creating an organ-on-a-chip, which they called “Liver-Chip,” that could be used to test drugs to see if they will cause liver damage. To that end, they created a device that emulates parts of the liver. The result was a sandwich of sorts, with a porous material in the middle covered by an extracellular matrix.
Underneath they created a parenchymal channel. They then added real human hepatocytes on top, and endothelial, Kupffer and stellate cells where they would be on a real liver. The device was then connected to pods using liquid-to-liquid connections to allow for emulating the human fluidic system in the liver. Then, they added several drugs, one by one, to see if they would interfere with any of the emulated liver parts, including the cells.
The team tested 870 versions of their device, using 27 drugs, many of which were already known to cause liver damage. They found that their Organ-Chips outperformed conventional clinical trial models—they identified 87% of the drugs that were known to cause liver damage despite having passed animal testing.
More information:
Lorna Ewart et al, Performance assessment and economic analysis of a human Liver-Chip for predictive toxicology, Communications Medicine (2022). DOI: 10.1038/s43856-022-00209-1
Journal information:
Communications Medicine
Source: Read Full Article