A new breast cancer test could detect women who are less likely to respond to a standard breast cancer treatment—and could help improve treatment for these women by giving them other treatment options earlier.
A research team led by scientists at The Institute of Cancer Research, London, has identified a set of new “biomarkers”—or biological markers—that are associated with poor response to drugs called aromatase inhibitors, in patients with so-called estrogen receptor positive, human epidermal growth factor receptor 2 positive (ER+/HER2+) breast cancers.
The findings are significant in their potential to guide future treatment for women with ER+/HER2+ tumors, and in providing an unprecedented insight into the molecular and genetic profiles of this tumor type.
Not all patients benefit from the same treatment
ER+/HER2+ breast cancer is a molecularly diverse disease and the intrinsic differences between the cancers of patients can lead to different responses to the same treatment. This makes it very challenging to select patients who may benefit from hormone therapy.
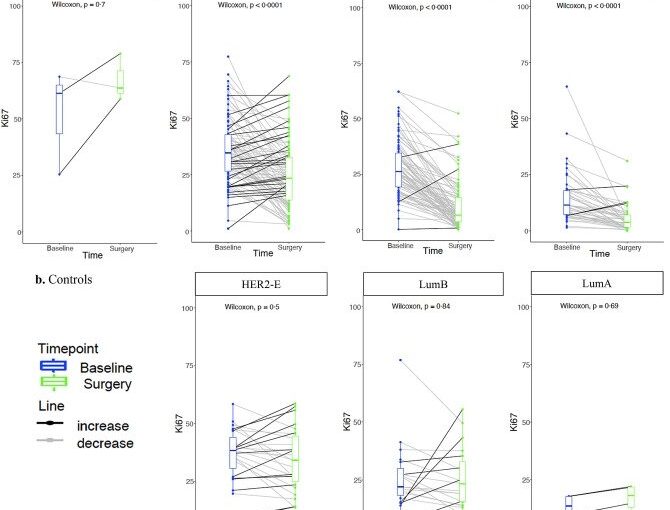
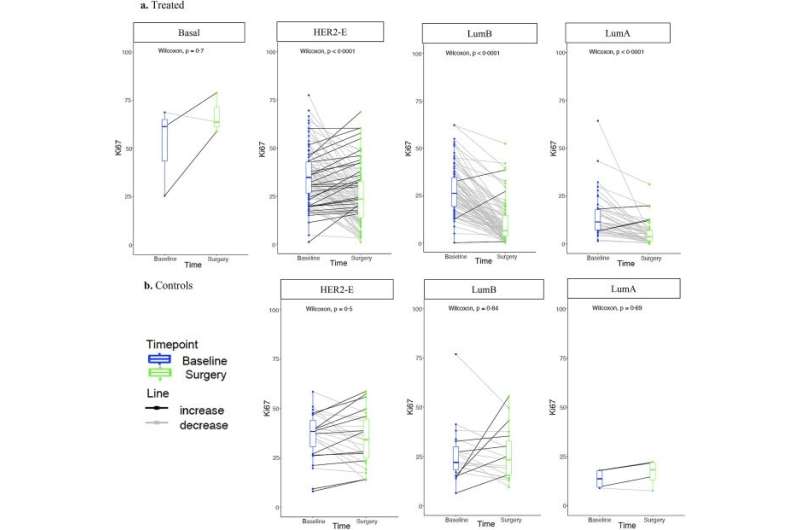
In a study published in eBioMedicine, the researchers genetically profiled more than 300 ER+/HER2+ breast cancer tumors and compared the levels of Ki67—a protein that marks cancer cell growth—in samples taken when patients were first diagnosed, and at two weeks after starting aromatase inhibitor treatment. They then applied advanced machine learning methods to group genetic and molecular features associated with good or poor response.
Tumor samples for the study were sourced from the phase III POETIC trial, which tested the benefit of two weeks of presurgical aromatase inhibitor therapy at reducing the risk of recurrence in women with early-stage, hormone-positive breast cancer.
The researchers identified five new subtypes of ER+/HER2+ tumors based on their molecular and genetic characteristics, each of which was associated with a different time to relapse after treatment.
One subtype in particular—which they called “HER2-enriched”—had a high rate of cancer cell growth and a relatively short time to relapse after treatment, indicating poor response to aromatase inhibitors.
Study leader Dr. Maggie Cheang and her team hopes to apply the findings from this study to develop more effective treatments for patients with ER+/HER2+ breast cancer. They have filed a patent for these new biomarkers and are now looking for opportunities to further progress the project with an industry partner.
The researchers are also looking for opportunities for collaboration on another project, which has uncovered biomarkers associated with resistance to a different type of breast cancer drug called CDK4/6 inhibitors and aromatase inhibitors.
Important tools for cancer research and care
Biomarker tests look for genetic, protein or imaging “markers” that provide doctors with crucial information about a patient’s disease. This helps doctors to identify patients with particular weaknesses in their cancers and match them to their optimal treatments.
However—as a consensus group convened by the ICR has warned—biomarker testing is not always available due to limited resources and lack of updated regulations.
The group, which included charities, stakeholder groups and life-science companies, has called for the NHS to offer all cancer patients genetic profiling of their tumors, both at diagnosis and during treatment, to improve personalized care and to track how the disease evolves.
Patients with breast cancer currently have their tumors classified using three “gold standard” biomarkers: the estrogen and progesterone hormone receptors and HER2, the presence of which on the surface of breast cancer cells guides the use of treatments including the drugs tamoxifen and trastuzumab, also known by its brand name Herceptin.
Characterizing breast cancer subgroups more closely
Study lead Dr. Maggie Cheang, leader of the Clinical Trials and Statistics Unit (ICR-CTSU) Integrative Genomic Analysis in Clinical Trials Team at The Institute of Cancer Research, London, said, “This is the first and largest study exploring mechanisms of resistance to hormone therapy in HER2 positive, estrogen receptor positive breast cancer. Our results establish our ‘HER2-enriched’ subtype as the first predictive biomarker associated with resistance to aromatase inhibitors and worse outcome in these breast cancers.
“Our study also highlights the need to characterize breast cancer subgroups more closely. Molecular profiling of tumors allows identification and development of new biomarkers that can increase our understanding of disease and treatment resistance.
“The hormone receptors and HER2 are established biomarkers that have been around for many years, and though they are still necessary, they are no longer enough. The field has seen rapid advances in recent years, so we need to update the biomarker testing and treatment guidelines accordingly to improve patient care.”
More information:
Milana A. Bergamino et al, HER2-enriched subtype and novel molecular subgroups drive aromatase inhibitor resistance and an increased risk of relapse in early ER+/HER2+ breast cancer, eBioMedicine (2022). DOI: 10.1016/j.ebiom.2022.104205
Journal information:
EBioMedicine
Source: Read Full Article