ROCENSPR11220
This medicine is subject to additional monitoring. This will allow quick identificationof new safety information. You can help by reporting any side effects you may get.You can report side effects to your doctor, or directly at www.tga.gov.au/reporting-problems .
satralizumab
Consumer Medicine Information
What is in this leaflet
This leaflet answers some common questions about Enspryng. It does not contain all the available information. It does not take the place of talking to your doctor or pharmacist.
All medicines have risks and benefits. Your doctor has weighed the risks of you using Enspryng against the benefits they expect it will have for you.
If you have any concerns about using this medicine, ask your doctor or pharmacist.
Keep this leaflet with the medicine.
You may need to read it again.
What Enspryng is used for
Enspryng contains the active ingredient satralizumab.
Satralizumab belongs to a group of medicines called monoclonal antibodies. Monoclonal antibodies are a type of protein which recognise and attach to a specific protein in the body.
Enspryng is used to treat neuromyelitis optica spectrum disorders (NMOSD) in adult patients, who test positive for an antibody called AQP4-IgG (aquaporin-4 immunoglobulin G), also known as NMO-IgG (neuromyelitis optica immunoglobulin G).
NMOSD is an autoimmune disease of the central nervous system. This is a condition where the immune system attacks the central nervous system. NMOSD mainly affects the optic nerves (nerves found in your eye) and spinal cord. The damage to the optic nerves causes inflammation – leading to pain and loss of sight. The damage to the spinal cord causes weakness or loss of movement in the legs or arms, loss of feeling, and problems with bladder and bowel function.
In a ‘relapse’, or an ‘attack’ of NMOSD, there is inflammation in the nervous system. This inflammation causes people to have new symptoms, or have symptoms that they have had before.
Enspryng works by blocking the action of a protein called ‘interleukin-6’ (IL-6). This protein is involved in inflammation in the body and the levels of this protein are high in NMOSD. Enspryng may reduce the risk of a relapse or attack of NMOSD.
Ask your doctor if you have any questions about why this medicine has been prescribed for you.
Your doctor may have prescribed it for another reason.
This medicine is available only with a doctor’s prescription.
Before you use Enspryng
If you are not sure if you should start receiving Enspryng, talk to your doctor.
When you must not use it
Do not use Enspryng:
if you have an allergy to satralizumab or any of the other ingredients listed at the end of this leaflet
if you have had an allergic reaction to any other medicine which contains proteins that are of hamster origin
Some of the symptoms of an allergic reaction may include:
shortness of breath
wheezing or difficulty breathing
swelling of the face, lips, tongue or other parts of the body
rash, itching or hives on the skin
Do not use Enspryng if the expiry date (EXP) printed on the pack has passed.
If you use this medicine after the expiry date has passed it may not work as well.
Do not use this medicine if you notice that it is cloudy, discoloured or contains visible particles. Enspryng is a colourless to slightly yellow liquid.
Do not use this medicine if the pack is torn or shows signs of tampering.
If it has expired or is damaged, return it to your pharmacist for disposal.
If you are not sure whether you should start using this medicine, talk to your doctor.
Before you start to use Enspryng
Tell your doctor or nurse straight away if you think you have any signs of infection before, during or after Enspryng treatment such as:
fever or chills
cough that does not go away
sore throat
herpes (such as cold sore, shingles or genital sores)
skin redness, swelling, tenderness or pain
feeling or being sick, diarrhoea or belly pain.
You cannot use Enspryng while you have an infection.
Your doctor will wait until the infection has resolved before starting or resuming treatment with Enspryng.
Tell your doctor if you have recently been given any vaccines or might be given vaccines in the near future.
Your doctor will check if you need any vaccines before you start Enspryng.
Do not have ‘live’ or ‘live attenuated’ vaccines while you are being treated with Enspryng (for example BCG for tuberculosis or vaccines against yellow fever).
You cannot receive Enspryng if you are having some vaccines (‘live’ or ‘live attenuated’).
Tell your doctor or nurse straight away if you have any of the following signs of increased liver enzymes during or after Enspryng treatment:
yellowing of the skin and the whites of the eyes (jaundice)
dark coloured urine
feeling and being sick
Enspryng can affect your liver function and increase the amount of some liver enzymes in your blood. Your doctor will do blood tests to check these amounts and monitor how well your liver is working.
Tell your doctor if you have allergies to any other medicines, foods, preservatives or dyes.
Tell your doctor if you are pregnant or are planning to have a baby. Enspryng is not recommended during pregnancy. Women of childbearing potential must use effective contraception during and up to three months after treatment with Enspryng.
However, if there is a need to use Enspryng when you are pregnant, your doctor will discuss the risks and benefits to you and your unborn baby.
Tell your doctor if you are breastfeeding or plan to breastfeed.
Your doctor may advise you to stop breastfeeding during treatment with Enspryng. It is not known if Enspryng passes into breast milk.
Enspryng is not recommended in children and adolescents under the age of 18 years.
Safety and efficacy in children and adolescents younger than 18 years have not been established.
Enspryng is not recommended in elderly patients over 74 years of age.
Safety and efficacy of Enspryng in elderly over 74 years of age have not been established.
If you have not told your doctor about any of the above, tell him/her before you start using Enspryng.
Taking other medicines
Tell your doctor or pharmacist if you are using any other medicines, including any that you get without a prescription from your pharmacy, supermarket or health food shop.
Your doctor and pharmacist have more information on medicines to be careful with or avoid while using this medicine.
How to use Enspryng
Follow all directions given to you by your doctor or pharmacist carefully.
They may differ from the information contained in this leaflet.
If you do not understand the instructions on the box, ask your doctor or pharmacist for help.
Enspryng is given by injection under the skin (subcutaneously).
At the start, your doctor or nurse may inject Enspryng. However, your doctor may decide that you or your adult caregiver can inject Enspryng.
You or your caregiver will get training on how to inject Enspryng.
Talk to your doctor or nurse if you or your caregiver have any questions about giving injections.
How much to use
Each injection contains 120 mg of satralizumab. Inject the entire content of the syringe each time.
How to use it
The first injection will be given under the supervision of your doctor or nurse.
The first three injections are given once every two weeks. These are called ‘loading doses’.
After this, the injection is given every four weeks. This is called the ‘maintenance dose’. Keep using Enspryng once every four weeks for as long as your doctor tells you to.
After removing the cap, the injection must be started within 5 minutes to prevent the medicine from drying out and blocking the needle. If the pre-filled syringe is not used within 5 minutes of cap removal, you must dispose of it in a sharps container and use a new pre-filled syringe.
Directions for self-injection
You or your caregiver should read these directions from beginning to end before starting to inject so that you or your caregiver are familiar with each step of the procedure. These instructions must be carefully followed.
Consult with your doctor if you or your caregiver require further instructions.
These instructions do not replace the instructions from your doctor.
Your doctor should show you how to prepare and inject properly before you or your caregiver inject for the first time.
Ask them any questions you or your caregiver may have.
Do not attempt to administer an injection until you or your caregiver understand how to self-inject Enspryng.
It is important to remain under your doctor’s care while using Enspryng. It is recommended you have someone else present when you self-inject Enspryng in case you experience any symptoms of a serious allergic reaction described under Before you use Enspryng, When you must not use it.
The syringe is for single use only and should be safely discarded after use.
How to inject using the syringe
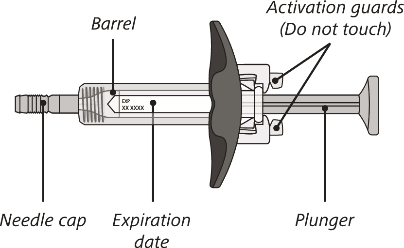
The syringe components:
Before use
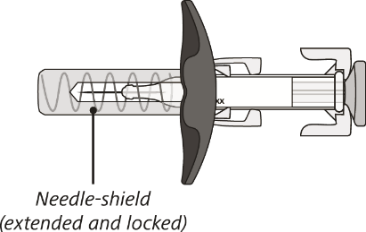
After use
The syringe has a needle-shield that automatically covers the needle when the injection is complete.
Do not shake the syringe.
Do not try to open the syringe or take it apart.
Do not re-use the same syringe.
Gather what you will need:
Included in the pack:
1 pre-filled syringe
Not included in the pack:
Alcohol pad
Sterile cotton ball or gauze
Sharps container for safe disposal of the needle cap and used syringe.
STEP 1: Preparing to use Enspryng
Take the carton containing the syringe out of the refrigerator and place it on a clean, flat work surface (like a table).
Check the expiry date on the back of the carton. Do not use if the carton has expired.
Check that the front of the carton is sealed. Do not use if the seal has been broken.
STEP 2: Removal of the pre-filled syringe from the carton
Open the sealed carton and carefully lift the syringe out of the carton by holding the barrel.
Do not turn the carton upside down to remove the syringe.
Do not touch the activation guards as this may damage the syringe.
Do not hold the plunger or needle cap.
STEP 3: Visual inspection of the pre-filled syringe
Check the expiration date on the syringe. Do not use the syringe if it has expired.
Check the syringe for any damage. Do not use if it is cracked or broken.
Check that the liquid through the viewing window is clear and colourless to slightly yellow.
Do not inject the medicine if the liquid is cloudy, discoloured, or has particles in it.
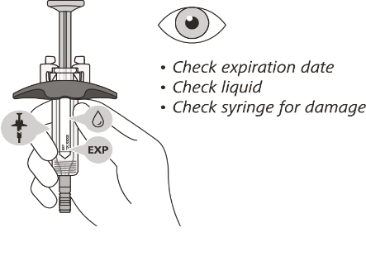
There may be some small air bubbles in the syringe. This is normal and you should not try to remove them.
STEP 4: Preparing to inject Enspryng
Let your syringe reach room temperature.
Place it on a clean, flat work surface (like a table) for 30 minutes to allow it to reach room temperature.
It is important to let the syringe gently warm up as injecting cold medicine may feel uncomfortable and make it harder to push.
Wash your hands with soap and water.
Choose your injection site in either:
the lower part of your stomach (abdomen) or
the front and middle of your thighs.
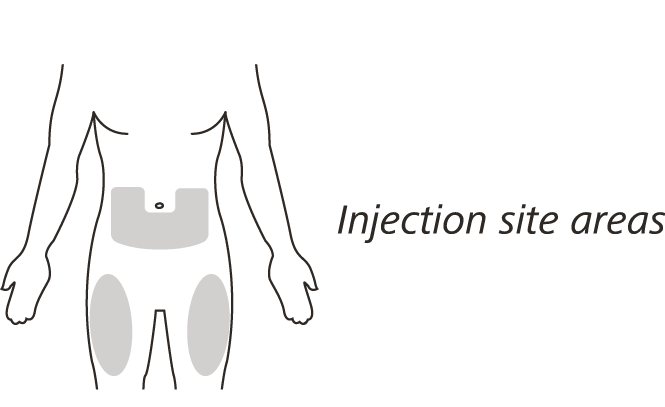
Do not inject into the 5 cm area around your belly button.
Do not inject into moles, scars, bruises, or areas where the skin is tender, red, hard or broken.
Choose a different injection site for each new injection – choose a different place to inject which is at least 2.5 cm away from the place where you last injected.
Clean the injection site by wiping it with an alcohol pad and let it air dry.
Do not fan or blow on the area which you have cleaned.
Do not touch the injection site again before you inject Enspryng.
STEP 4: Injecting Enspyng
Hold the barrel of the syringe between your thumb and index finger. With your other hand, pull the needle cap straight off.
You may see a drop of liquid at the end of the needle – this is normal and will not affect your dose.
Use the syringe within 5 minutes of removing the cap or the needle may clog.
Do not take the needle cap off until you are ready to inject Enspryng.
Do not put the needle cap back on once it has been removed as this may damage the needle.
Do not touch the needle or let it touch any surfaces after removing the needle cap.
Hold the barrel of the syringe using your thumb and index finger. With your other hand, pinch the area of skin you have cleaned.
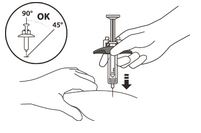
Use a quick, dart-like motion to insert the needle at an angle between 45° to 90°
Do not insert the needle through clothing.
Do not change the angle of the injection.
Do not insert the needle again.
After the needle is inserted, let go of the pinched skin.
Slowly inject all of the medicine by gently pushing the plunger all the way down until it touches the activation guards.
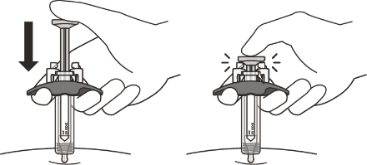
Gently release the plunger and allow the needle to come out of the skin at the same angle it was inserted.
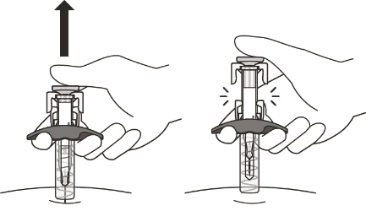
The needle will now be covered by the needle-shield. If the needle is not covered, carefully place the syringe into a sharps container to avoid injury.
STEP 5: Taking care of the injection site
There may be a little bleeding at the injection site. You can press a cotton ball or gauze over the injection site but do not rub it. If needed, you may also cover the area you injected with a small bandage. If the medicine gets into contact with your skin, wash the area with water.
STEP 6: Disposing of Enspryng
Do not try to re-cap your syringe.
Put your used syringe in a sharps container immediately after use.
Do not throw away (dispose of) the syringe in your household waste and do not recycle them.
Ask your doctor or pharmacist for information about where you can get a sharps container or what other types of puncture-resistant containers you can use to safely dispose of your used syringes and needle caps.
When to use it
It does not matter if you use Enspryng before or after food.
How long to use it
Continue using Enspryng for as long as your doctor tells you.
This medicine helps to control your condition, but does not cure it. It is important to keep using your medicine even if you feel well.
Do not suddenly stop using Enspryng without asking your doctor first. If you have any further questions on the use of this medicine, ask your doctor, pharmacist or nurse.
If you forget to use it
If you missed a dose of Enspryng, talk to your doctor on how to continue Enspryng treatment.
Do not inject a double dose on the same day to make up for a forgotten dose.
This may increase the chance of you
getting an unwanted side effect.
If you are not sure what to do, ask your doctor or pharmacist.
If you have trouble remembering to use your medicine, ask your pharmacist for some hints.
If you use too much (overdose)
Immediately telephone your doctor or the Poisons Information Centre (telephone 13 11 26) for advice, or go to Accident and Emergency at the nearest hospital, if you think that you or anyone else may have used too much Enspryng.
Do this even if there are no signs of discomfort or poisoning.
You may need urgent medical attention.
While you are using Enspryng
Things you must do
If you are about to be started on any new medicine, remind your doctor and pharmacist that you are using Enspryng.
Tell any other doctors, dentists, and pharmacists who treat you that you are using this medicine.
If you are having surgery, tell the surgeon or anaesthetist that you are using this medicine.
It may affect other medicines used during surgery.
If you are having any blood tests, tell your doctor that you are using this medicine.
It may interfere with the results of some tests.
If you develop a serious infection while using Enspryng, tell your doctor immediately.
You may need to stop using Enspryng until the infection is controlled.
Keep all of your doctor’s appointments so that your progress can be checked.
Things you must not do
Do not use Enspryng to treat any other complaints unless your doctor tells you to.
Do not give your medicine to anyone else, even if they have the same condition as you.
Do not stop using your medicine or lower the dosage without checking with your doctor.
Things to be careful of
Be careful driving or operating machinery until you know how Enspryng affects you.
This medicine is not expected to affect your ability to drive a car or operate machinery.
Side effects
Tell your doctor or pharmacist as soon as possible if you do not feel well while you are using Enspryng.
This medicine helps most people with NMOSD, but it may have unwanted side effects in a few people.
All medicines can have side effects. Sometimes they are serious, most of the time they are not. You may need medical attention if you get some of the side effects.
Ask your doctor or pharmacist to answer any questions you may have.
Do not be alarmed by the following lists of side effects.
You may not experience any of them.
During or after the injection
If any of the following happen during or after the injection, particularly in the first 24 hours after the injection, tell your doctor immediately or go to Accident and Emergency at your nearest hospital:
redness, itching, pain or swelling where the injection is given
rash, red or itchy skin or hives
feeling flushed
headache
throat irritation, swelling or pain
feeling short of breath
low blood pressure
fever or chills
feeling tired or dizzy
feeling or being sick or diarrhoea
fast heart rate, fluttering or pounding heart (heart palpitations).
The above list includes very serious side effects. You may need urgent medical attention or hospitalisation.
Tell your doctor or pharmacist if you notice any of the following and they worry you:
headache
joint pain
stiffness
migraine
being unable to sleep (insomnia)
swelling in your lower legs, feet or hands
rash or itching
allergies or hay fever
low fibrinogen (a protein involved in blood clotting) levels in the blood shown in tests.
This is not a complete list of all possible side effects. Your doctor or pharmacist has a more complete list. Others may occur in some people and there may be some side effects not yet known.
Tell your doctor or pharmacist if you notice anything that is making you feel unwell.
Some side effects can only be found when your doctor does blood tests from time to time to check your progress (for example, decreased white blood cell counts and increased bilirubin levels).
After using Enspryng
Storage
Keep your Enspryng pre-filled syringe in the carton until it is time to use it.
Store your Enspryng pre-filled syringe at 2°C – 8°C (Refrigerate. Do not freeze). Protect from light and moisture.
Do not shake.
Do not use the syringe if it has been frozen.
If stored at room temperature, the total time out of refrigeration should not be longer than 8 days at a temperature that does not exceed 30°C.
Do not store Enspryng or any other medicine in the bathroom or near a sink. Do not leave it on a window sill or in the car.
Keep it where children cannot reach it.
A locked cupboard at least one-and-a-half metres above the ground is a good place to store medicines.
Disposal
If your doctor tells you to stop using this medicine or the expiry date has passed, ask your doctor or pharmacist what to do with any medicine that is left over.
Product description
What it looks like
Each pack of Enspryng contains 1 pre-filled syringe which contains a colourless to slightly yellow liquid.
Ingredients
Enspryng contains 120 mg of satralizumab as the active ingredient.
It also contains:
histidine
aspartic acid
arginine
poloxamer
water for injections
Satralizumab is made using Chinese hamster ovary cells.
This medicine does not contain lactose, sucrose, gluten, tartrazine or any other azo dyes.
Distributor
Enspryng is distributed in Australia by:
Roche Products Pty Ltd
ABN 70 000 132 865
Level 8, 30-34 Hickson Road
Sydney NSW 2000
Medical enquiries: 1800 233 950
Please check with your pharmacist for the latest Consumer Medicine Information (CMI).
Australian Registration Number:
AUST R 326047
This leaflet was prepared in December 2020.







